The Solubilities of KF, NaF, and NH4F in H2, Their Respective Hydroxides ... - Earl W. Mautz - Google Books

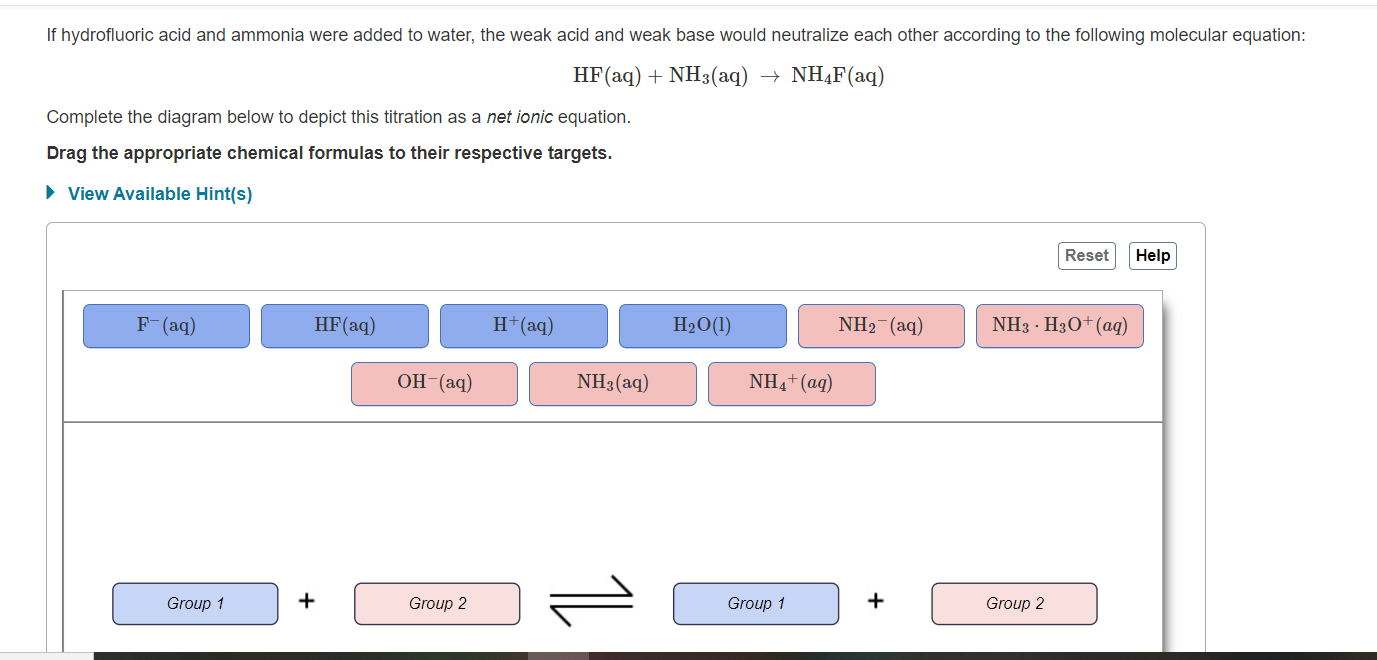
Will NH4F be acidic, basic or neutral in water? GIven: pKb NH3=4.75. KaHF=6.6*10^-4. I found for NH4 - YouTube

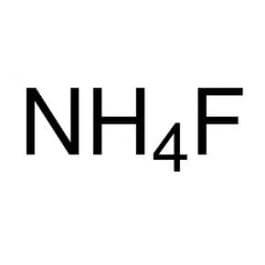
molécule de fluorure d'ammonium NH4F. Formule : image vectorielle de stock (libre de droits) 1946544001 | Shutterstock
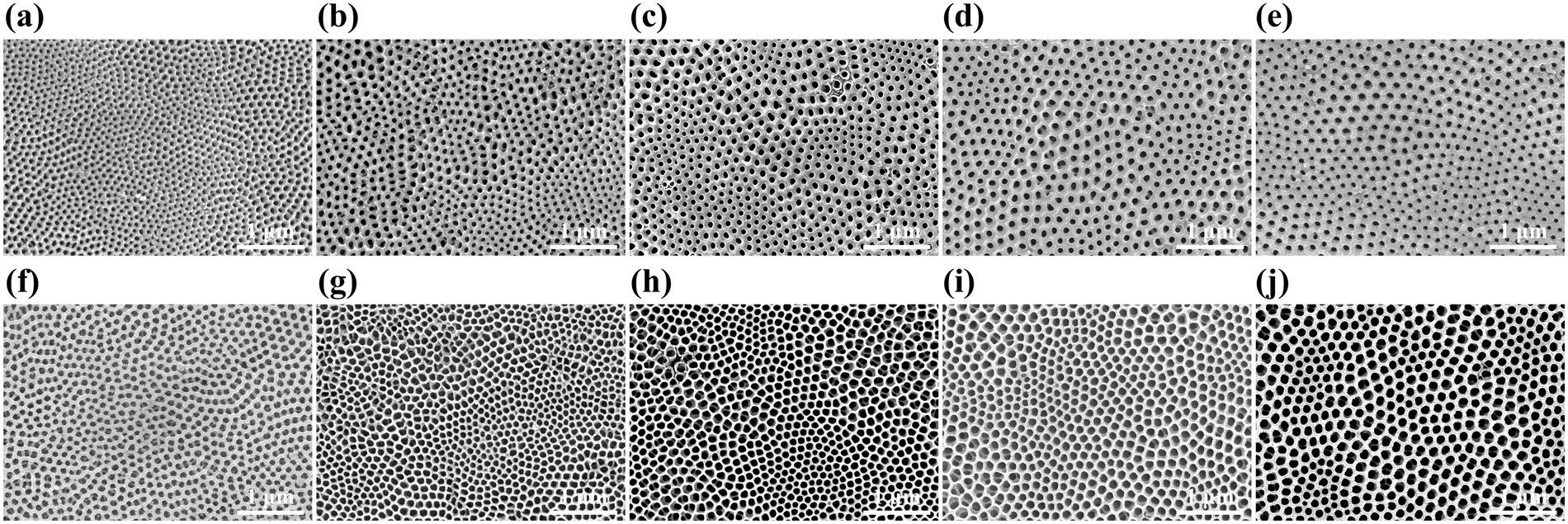
Effects of NH4F and distilled water on structure of pores in TiO2 nanotube arrays | Scientific Reports
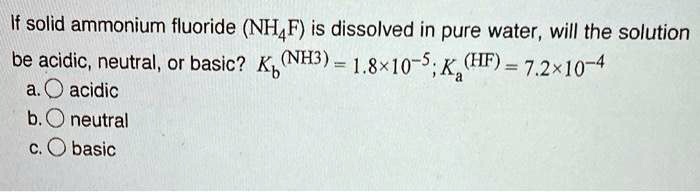
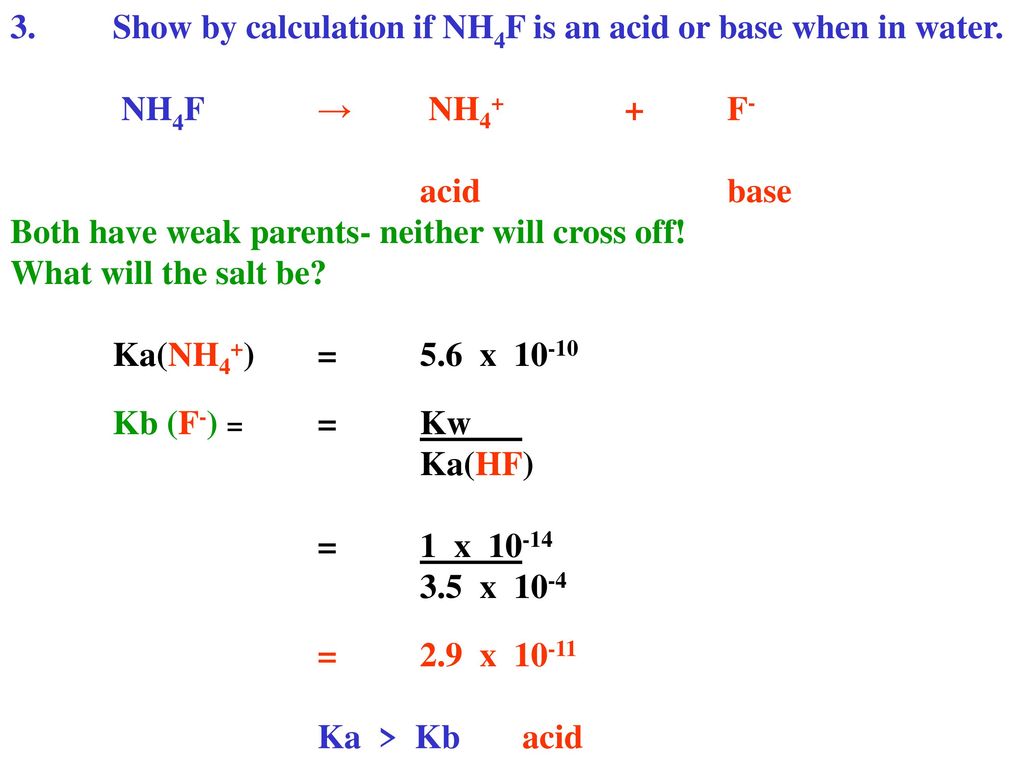
SOLVED: If solid ammonium fluoride (NH4F) is dissolved in pure water, will the solution be acidic, neutral, or basic? K(NH4F) = 1.8*10^-10. a. Acidic b. Neutral c. Basic.




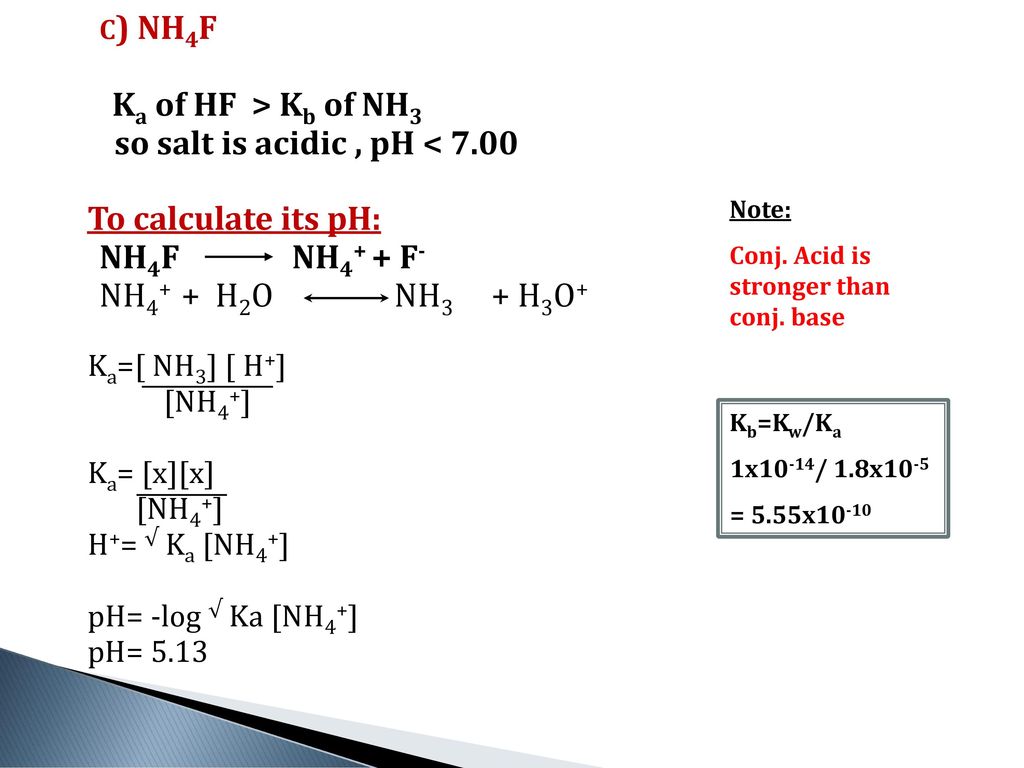

![Ammonium fluoride - Optional[19F NMR] - Chemical Shifts - SpectraBase Ammonium fluoride - Optional[19F NMR] - Chemical Shifts - SpectraBase](https://spectrabase.com/api/spectrum/BxPyFt2FJDU/structure.png?h=300&w=382)