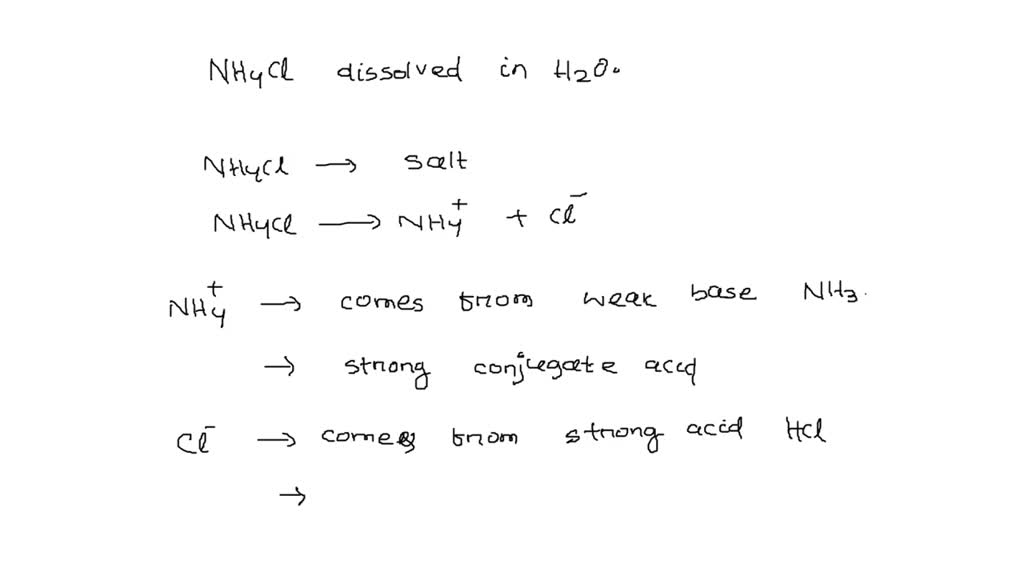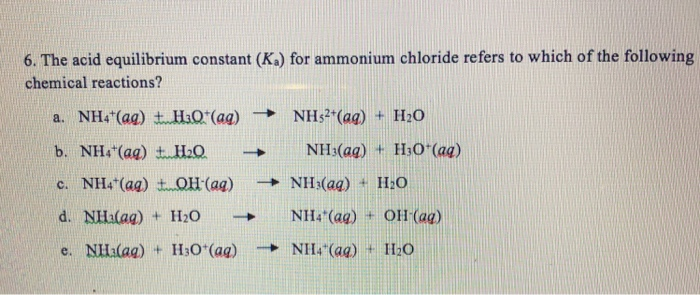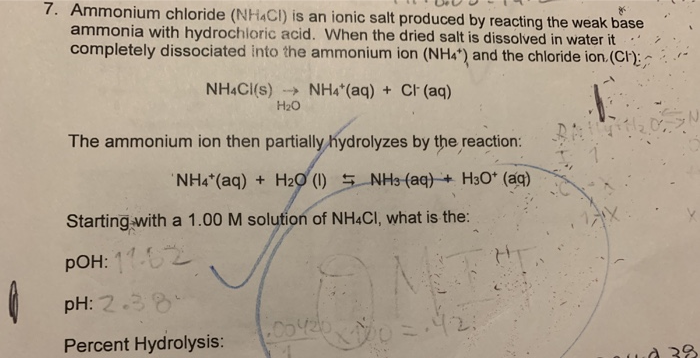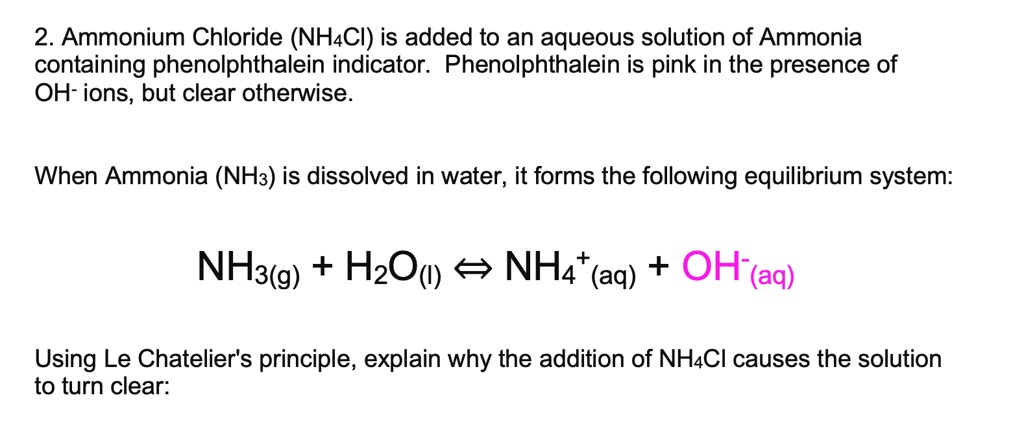
SOLVED: Ammonium Chloride (NH4Cl) is added to an aqueous solution of Ammonia containing phenolphthalein indicator. Phenolphthalein is pink in the presence of OH- ions, but clear otherwise. When Ammonia (NH3) is dissolved

The dissolution of ammonium chloride in water is an endothermic process but still it dissolves in - YouTube
![TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram](https://www.researchgate.net/publication/316335567/figure/fig4/AS:941601337643035@1601506661539/TG-and-DTG-of-CoEDTANH3Cl-H2O-a-TG-and-DSC-of-PTh-b-and-TG-and-DTG-of.gif)
TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram
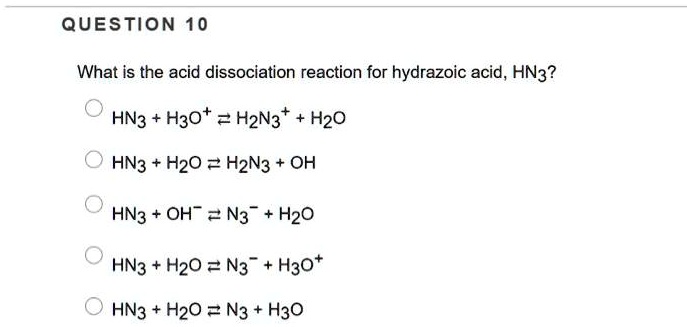
SOLVED: What is the acid dissociation reaction for hydrazoic acid, HN3? HN3 + H2O = H2N3 + H2O HN3 + H2O = H2N3 + OH HN3 + OH- = N3- + H2O HN3 + H2O = N3- + H3O+ HN3 + H2O = N3- + H3O+
![TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram](https://www.researchgate.net/profile/M-Dar/publication/316335567/figure/fig2/AS:941601337659404@1601506661415/XRD-pattern-of-CoEDTANH3Cl-H2O-a-and-PTh-CoEDTANH3Cl-H2O-nanocomposite-b_Q320.jpg)
TG and DTG of [Co(EDTA)NH3Cl] H2O (a); TG and DSC of PTh (b) and TG and... | Download Scientific Diagram

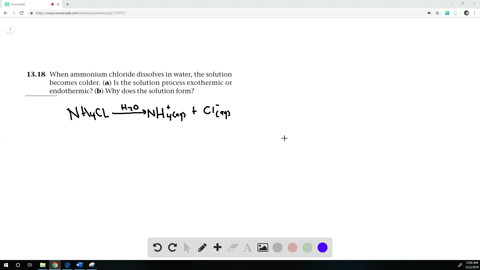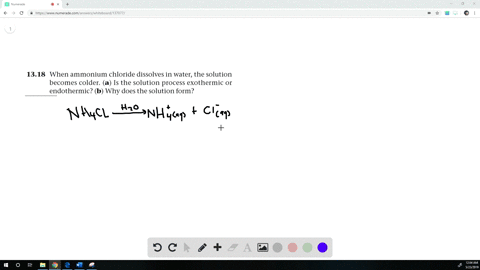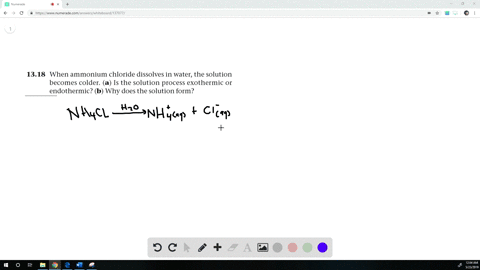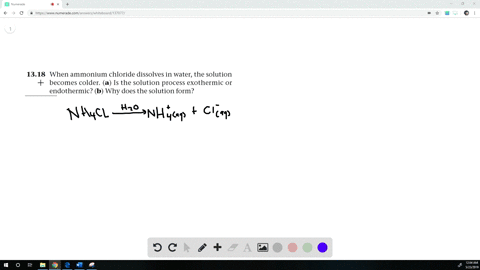
The equation for dissolving crystals of ammonium chloride in water at SLC is: NH4Cl(s) + H2O NH4+(aq) + Cl (aq); DH = 15.15 kJ/mol. Is this reaction endothermic or exothermic? | Homework.Study.com