
Revealing the Chemical Mechanism of NaO2 Decomposition by In Situ Raman Imaging | Chemistry of Materials
![PDF] Revision of the NaBO2–H2O phase diagram for optimized yield in the H2 generation through NaBH4 hydrolysis | Semantic Scholar PDF] Revision of the NaBO2–H2O phase diagram for optimized yield in the H2 generation through NaBH4 hydrolysis | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e8a022f49dd8cefe422671bdbf90195d1a22c0ee/26-Figure3-1.png)
PDF] Revision of the NaBO2–H2O phase diagram for optimized yield in the H2 generation through NaBH4 hydrolysis | Semantic Scholar
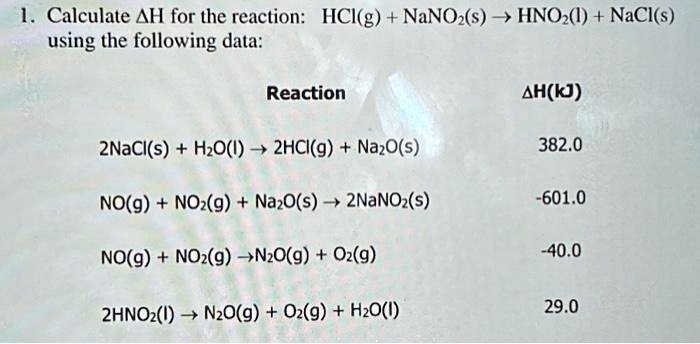
SOLVED: 1. Calculate ΔH for the reaction HCl(g) + NaNO2(s) → HNO2(l) + NaCl(s) using the following data: Reaction ΔH (kJ) 2NaCl(s) + H2O(l) → 2HCl(g) + Na2O(s) 382.0 NO(g) + NO(g) +

Highly Homogeneous Sodium Superoxide Growth in Na–O2 Batteries Enabled by a Hybrid Electrolyte | ACS Energy Letters
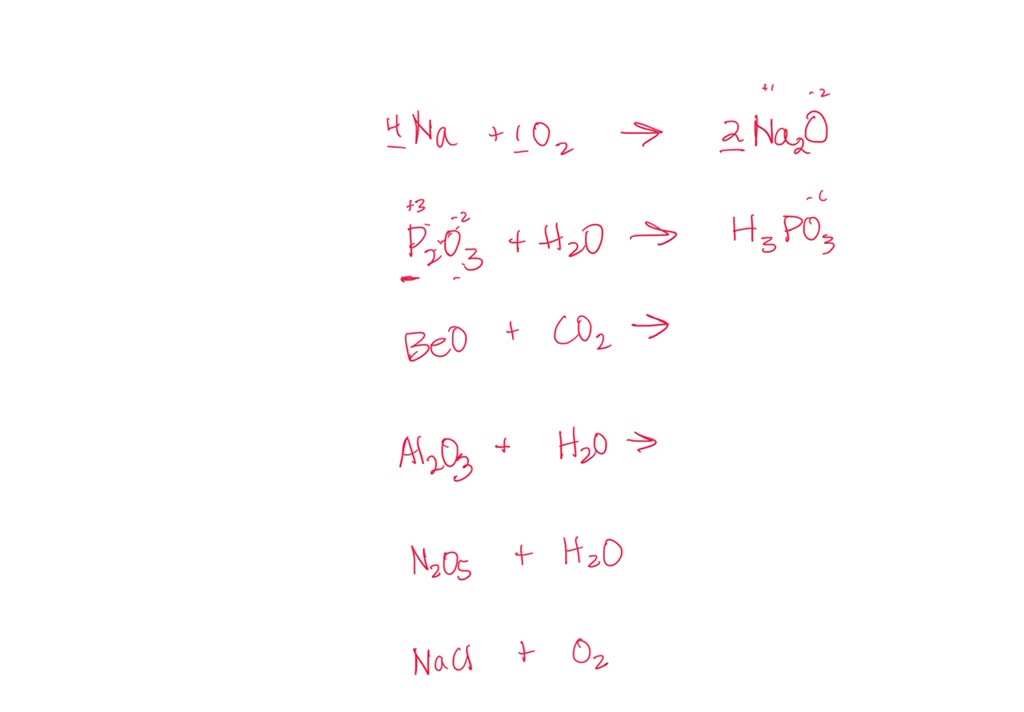
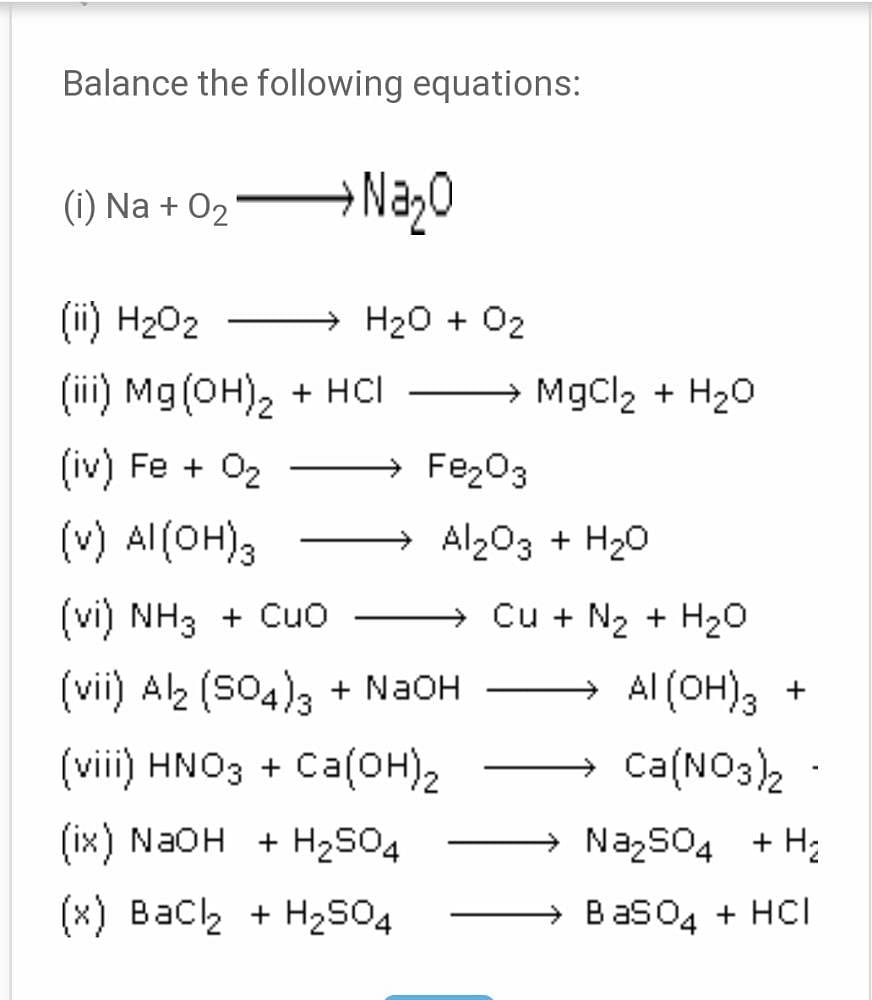
SOLVED: Identify the products of these synthesis reactions and balance the reactions: 6. Na + O2 → 7. P2O3 + H2O → 8. BeO + CO2 → 9. Al2O3 + H2O →

A Highly Stable All‐Solid‐State Na–O2/H2O Battery with Low Overpotential Based on Sodium Hydroxide (Adv. Funct. Mater. 41/2022) - Jiang - 2022 - Advanced Functional Materials - Wiley Online Library
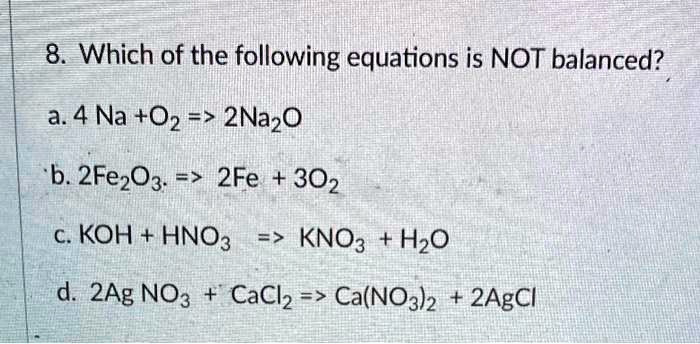



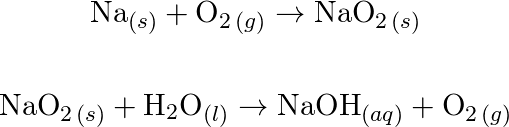
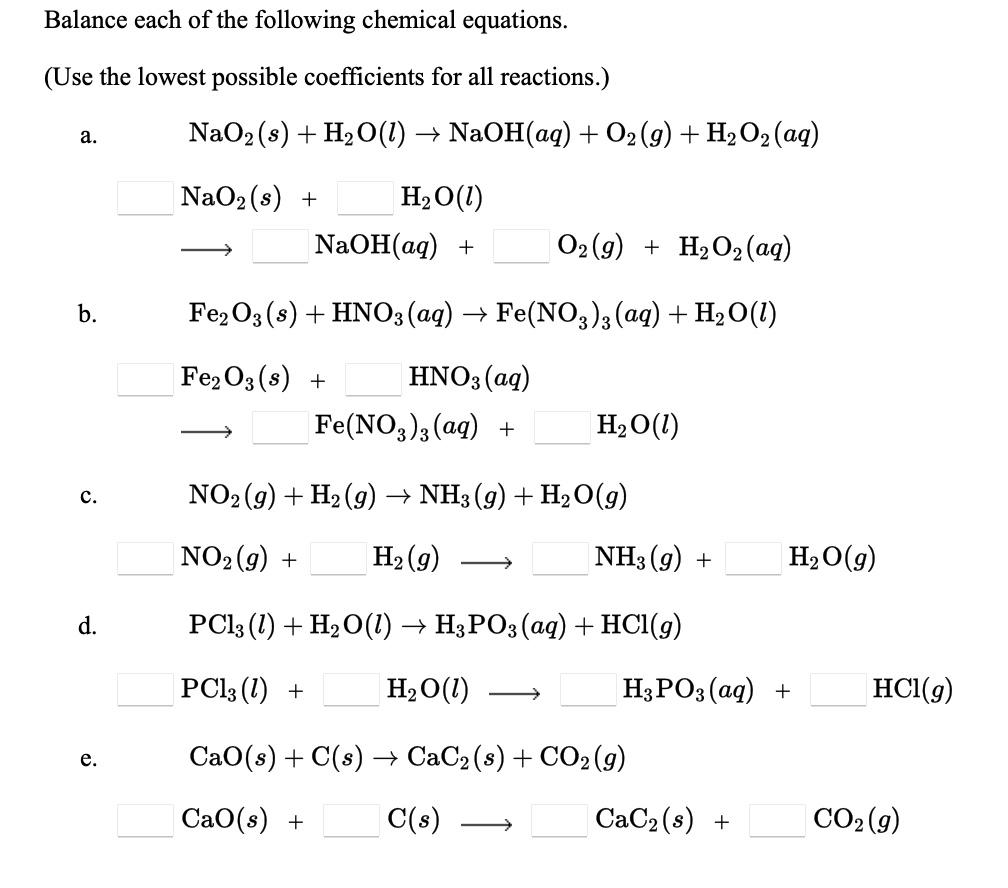
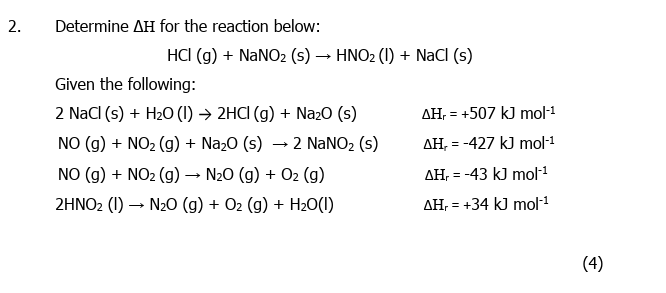
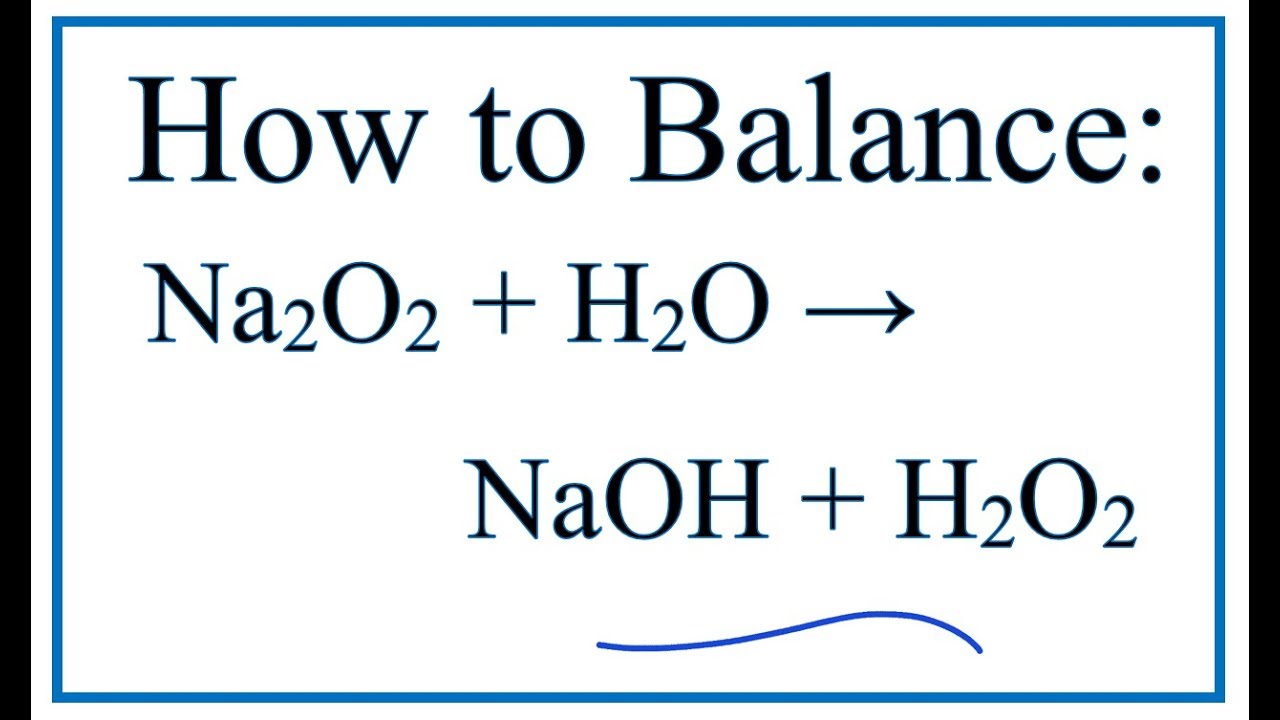





![Bengali] Balance the equation by oxidation no method :AI + NaOH + H2O Bengali] Balance the equation by oxidation no method :AI + NaOH + H2O](https://static.doubtnut.com/ss/web-overlay-thumb/8881612.webp)
