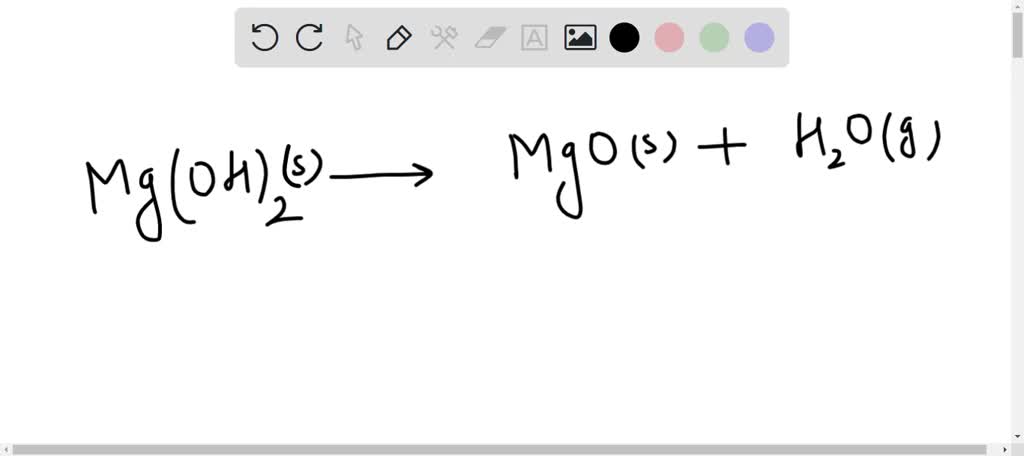
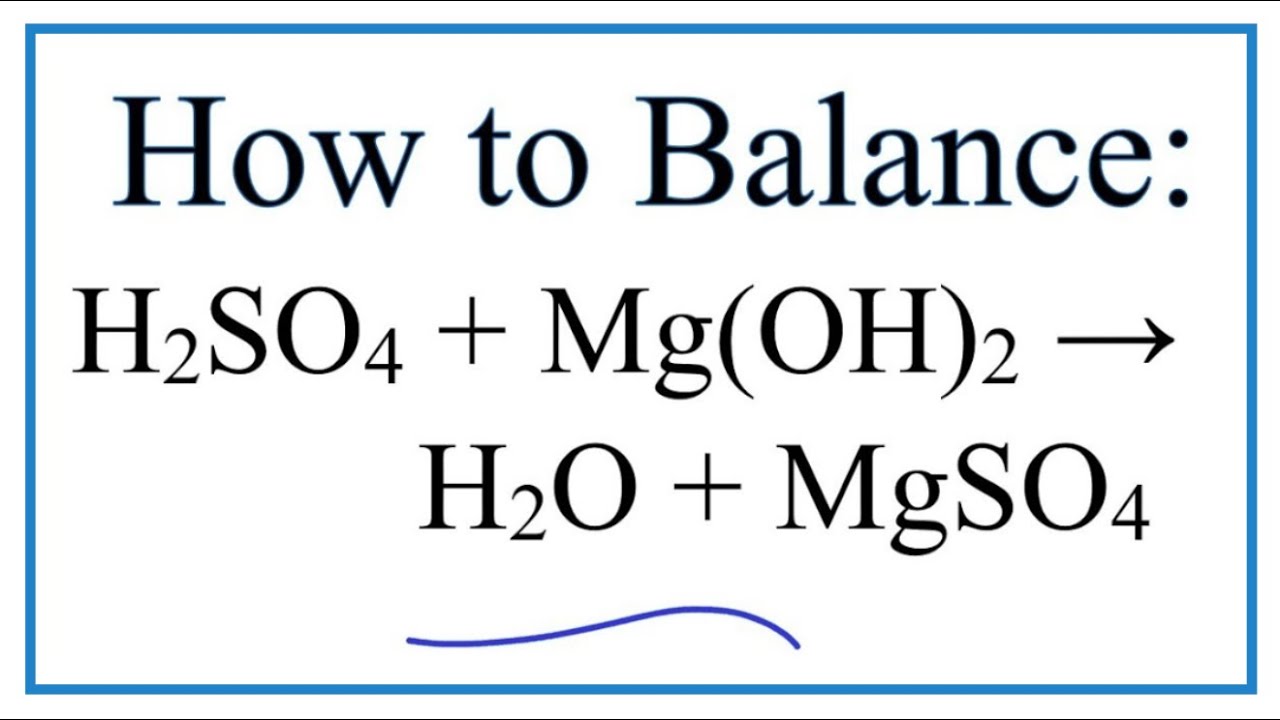
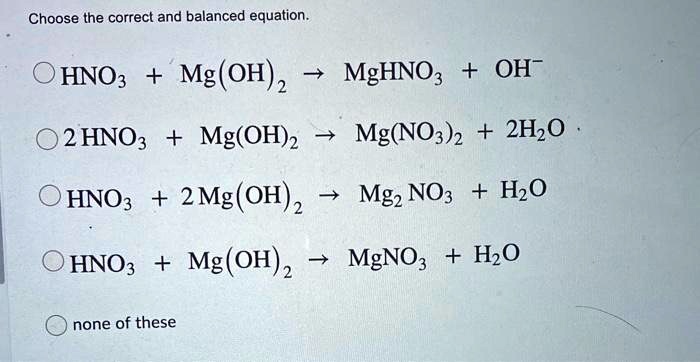
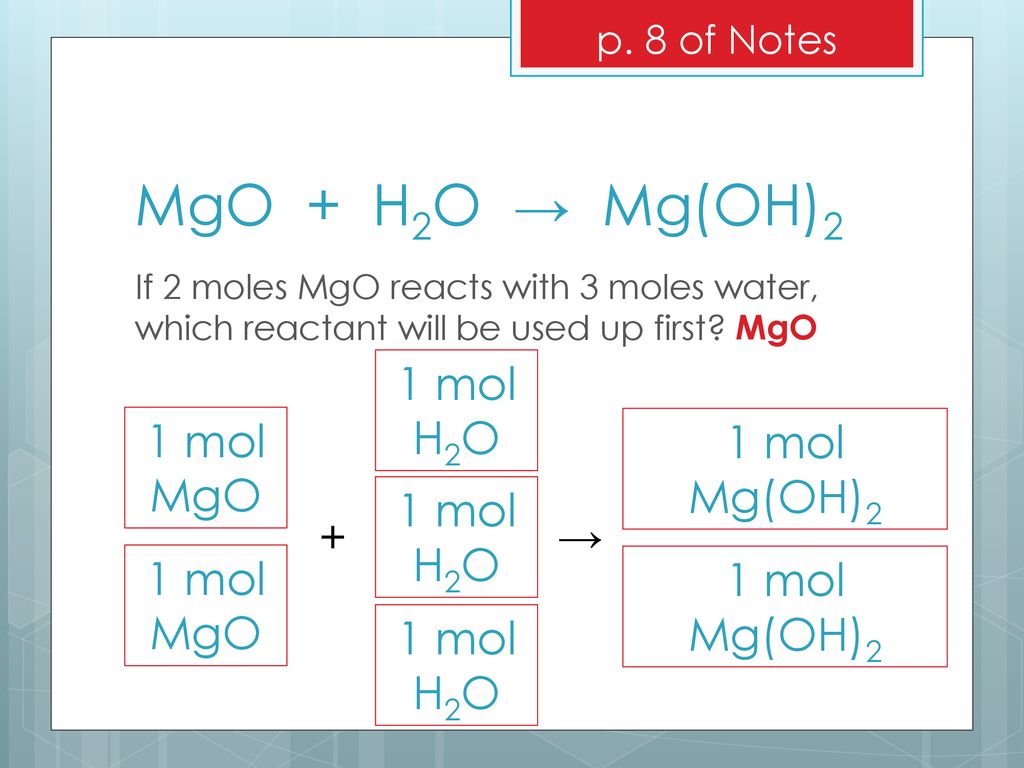
![SOLVED: How to set up the Ksp equilibrium expression for the dissolving of Magnesium Hydroxide in water? Mg(OH)2(s) + H2O(l) ⇌ Mg2+(aq) + 2OH-(aq) [Mg2+][OH-]2 ⇌ Mg(OH)2(s) Ksp = [Mg2+][OH-]2 SOLVED: How to set up the Ksp equilibrium expression for the dissolving of Magnesium Hydroxide in water? Mg(OH)2(s) + H2O(l) ⇌ Mg2+(aq) + 2OH-(aq) [Mg2+][OH-]2 ⇌ Mg(OH)2(s) Ksp = [Mg2+][OH-]2](https://cdn.numerade.com/ask_images/3e085c983b484497a99b9c58ad1fe940.jpg)
SOLVED: How to set up the Ksp equilibrium expression for the dissolving of Magnesium Hydroxide in water? Mg(OH)2(s) + H2O(l) ⇌ Mg2+(aq) + 2OH-(aq) [Mg2+][OH-]2 ⇌ Mg(OH)2(s) Ksp = [Mg2+][OH-]2

a The mechanism of Mg(OH)2 formation by means of the ionic exchange... | Download Scientific Diagram

How to Balance Mg(OH)2 + HCl = MgCl2 + H2O (Magnesium Hydroxide + Hydrochlo | Balancing equations, Equations, Molecules
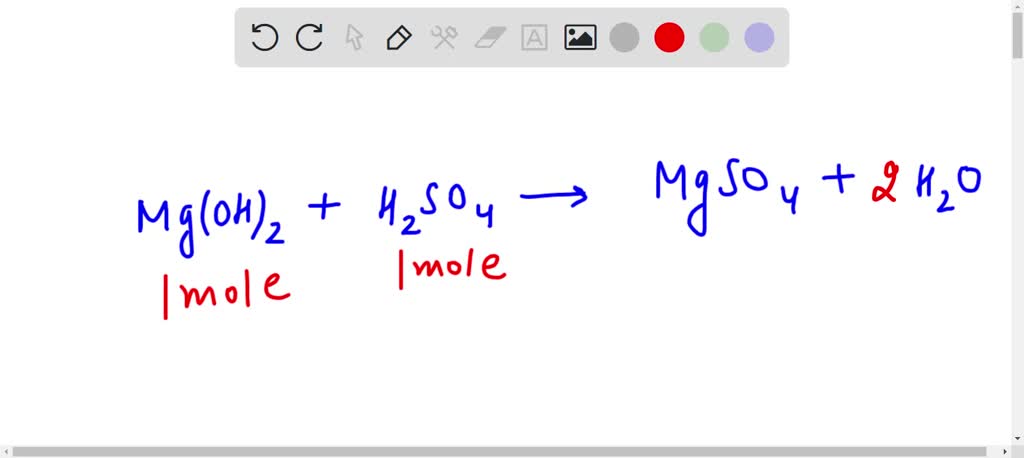
SOLVED: How many moles of H2O are produced when 1 mole of Mg(OH)2 reacts with 1 mole of H2SO4? I know the answer is 2 but I have to show work with

Solubility Equilibria in the System Mg(OH)2–MgCl2–H2O from 298 to 393 K | Journal of Chemical & Engineering Data