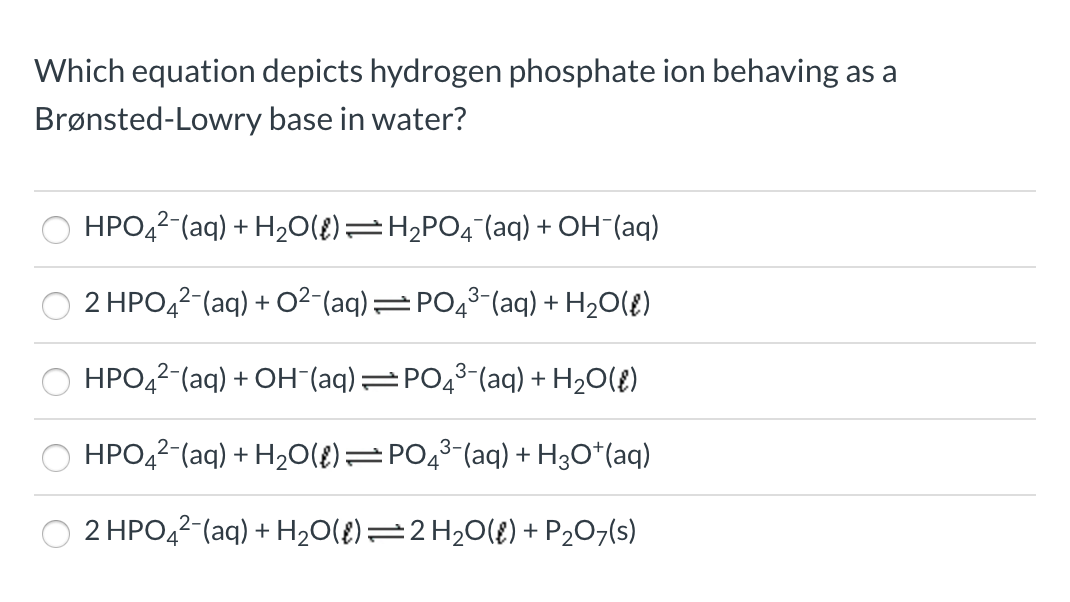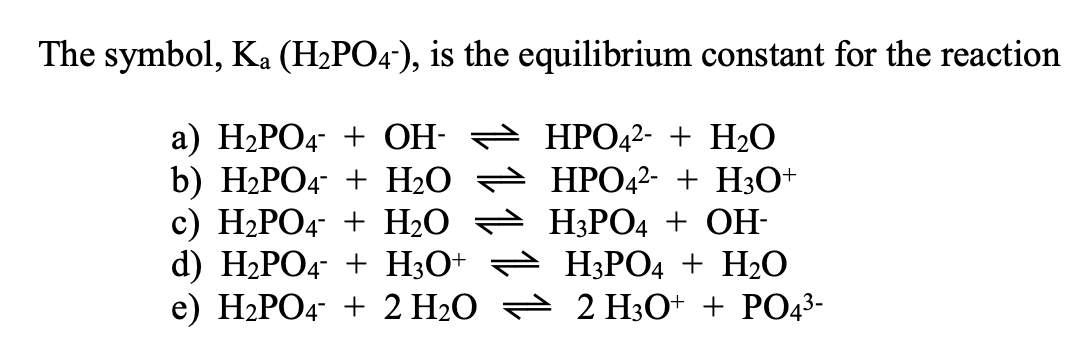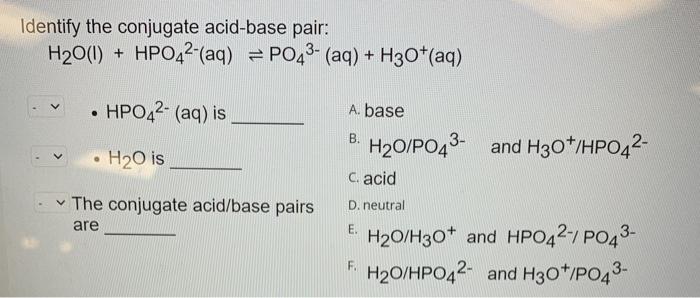PDF) Neutron powder diffraction study of α-Ti(HPO4)2.H2O and α-Hf(HPO4)2.H2O; H-atom positions | Pilar Pertierra - Academia.edu
![Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c03308/asset/images/medium/ic2c03308_0004.gif)
Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries
![Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.1c02685/asset/images/large/ic1c02685_0008.jpeg)
Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry

SOLVED: Choose balanced equation for the transfer of a proton between dihydrogen phosphate ion and the hydroxide ion: H2PO4-(aq) + OH-(aq) â†' HPO4 2-(aq) + H2O(l)

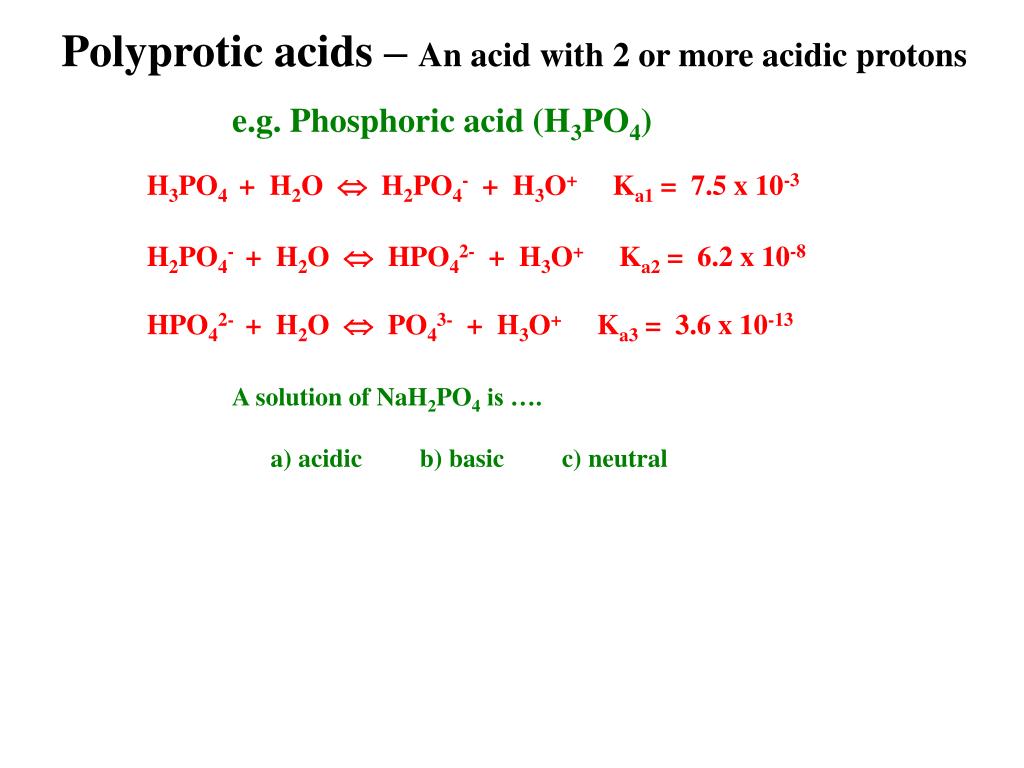
SOLVED: 7. Consider the following equilibrium equations: HPO4 + H2O ↔ H2PO4- + OH- H2PO4- + H2O ↔ HPO4 2- + H3O+ HPO4 2- + H2O ↔ H2PO4- + OH- (1) (2) (
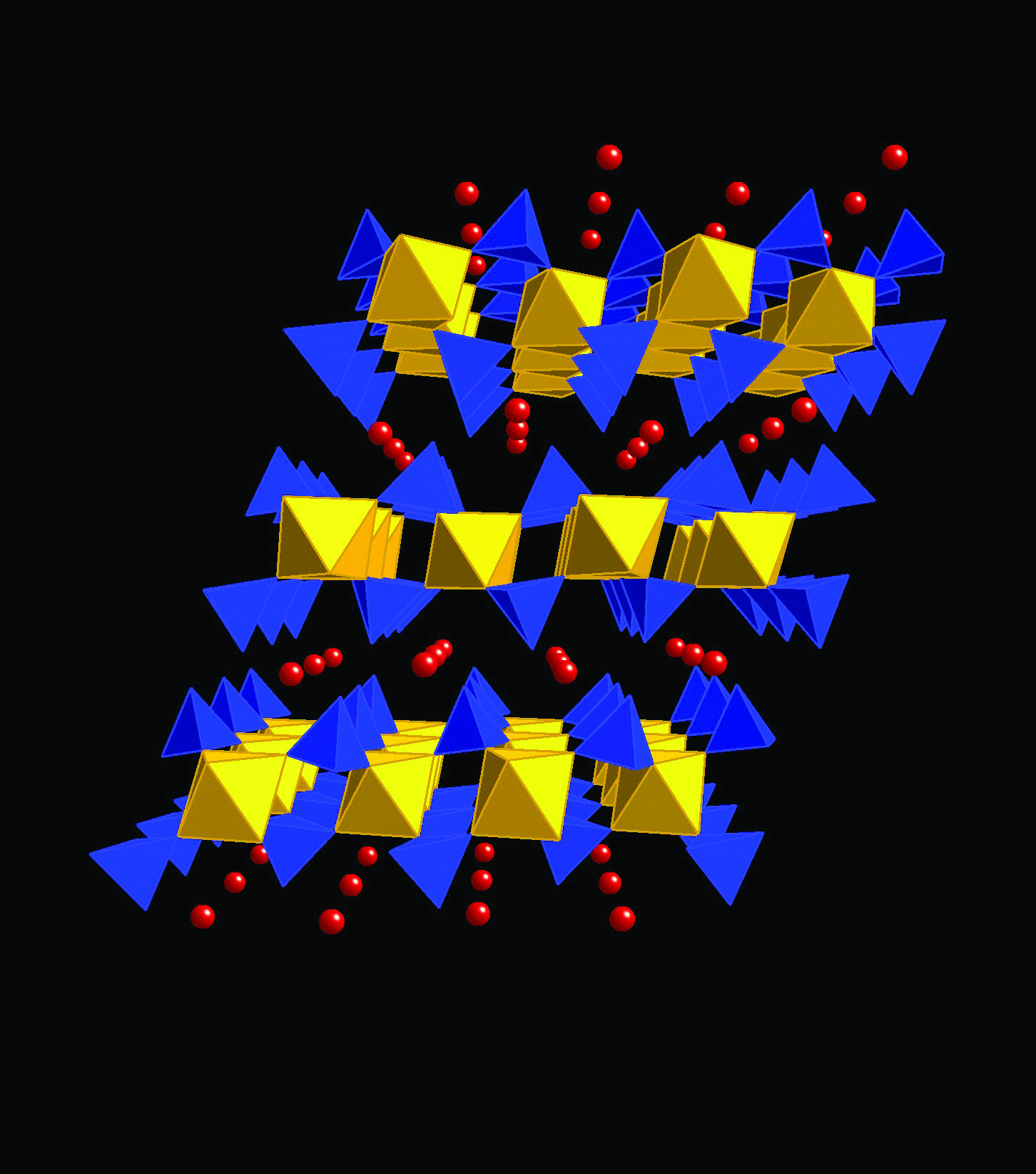
High resolution powder diffraction studies of mixed-metal layered phosphates - - Diamond Light Source

Catalytic conversions of isocyanate to urea and glucose to levulinate esters over mesoporous α-Ti(HPO4)2·H2O in green media - X-MOL
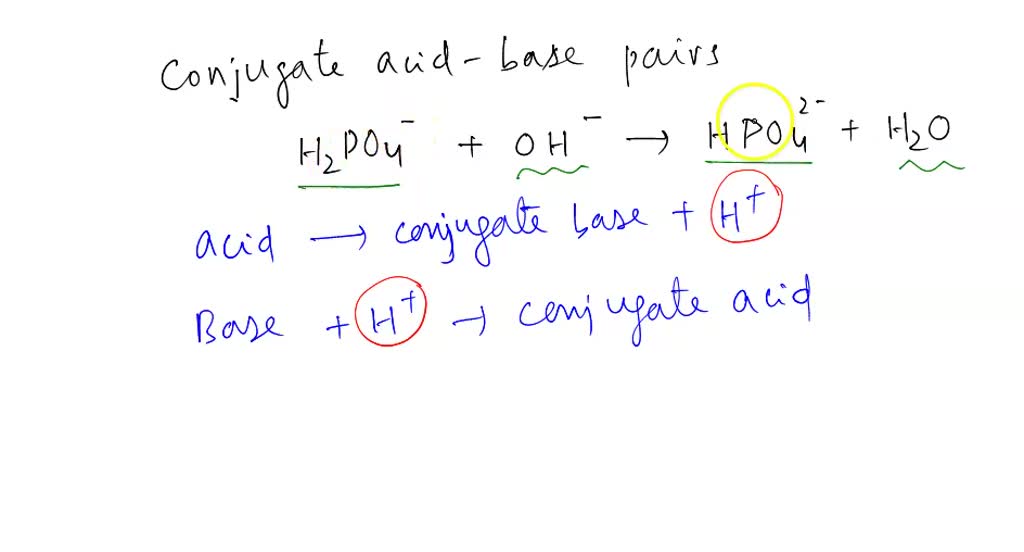
SOLVED: B. Identify the conjugate acid-base pairs in the following reactions: H2PO4- + OH- → HPO4-2 + H2O HBr + H2O → H3O+ + Br- CO3-2 + H2O → HCO3- + OH-
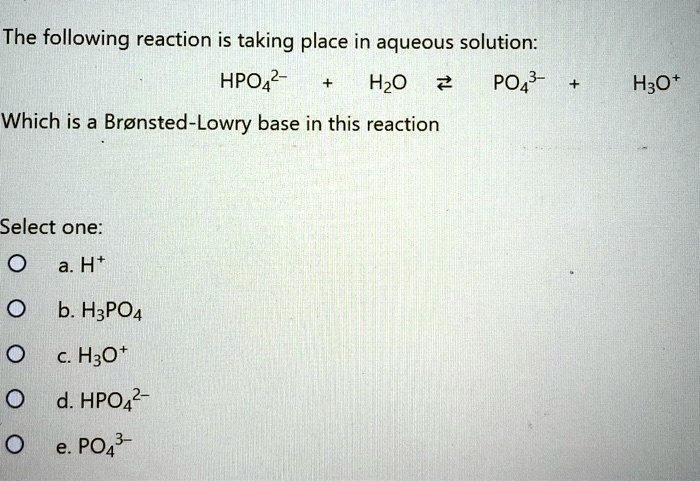
![Answered: What is the [HPO4-2] of a solution… | bartleby Answered: What is the [HPO4-2] of a solution… | bartleby](https://content.bartleby.com/qna-images/answer/25d6589c-34c4-49fd-a0de-f621c4bd4dbc/9f74c13f-f553-447b-8735-1f1f7effb02f/x03vih.png)