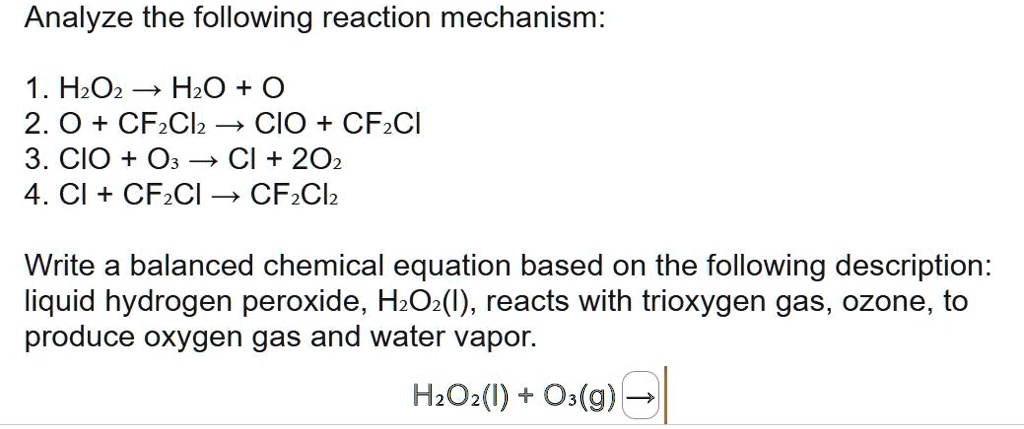
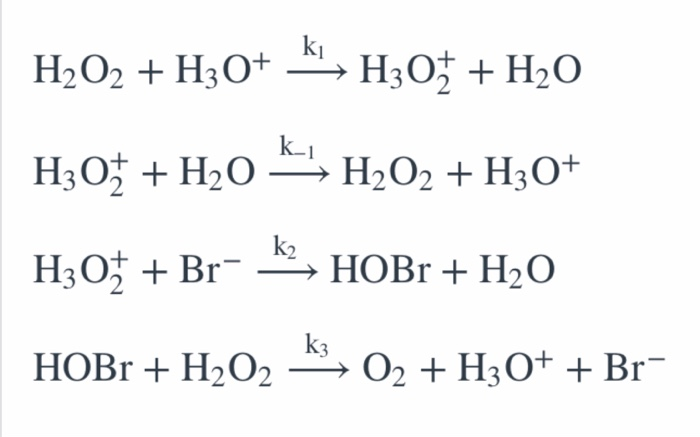Balance the following chemical equation H2O2+O3⇒H2O+O2 Indicating the changes in oxidation numbers of oxygen, the equivalent weight of H2O2 this reaction.

H2O2 is decomposed to H2O and O2 in following sequence of reaction. 1)H2O2(aq)+ I (aq) + H2O(0) + OI (aq) 2)H2O2(aq) +Ol'(aq) → H2O0) + O2(g) a) Write the chemical equation overall

H2O2=H2O+O2 balance the chemical equation @mydocumentary838. h2o2=h2o+o2 balance the equation. - YouTube

2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the

SOLVED: Equations: MnO4 + H2O2 + H2O -> MnO2 + O2 + H2O 2 MnO4 + 6 H2O2 + 5 H2O -> 2 MnO2 + 5 O2 + 8 H2O MnO4 + 8 H+ + Se -> MnO2 + 4 H2O O2 + 3 O2 + 2 e Mn7+ + 2 e -> Mn2+ Mn7+ + Se -> Mn2+
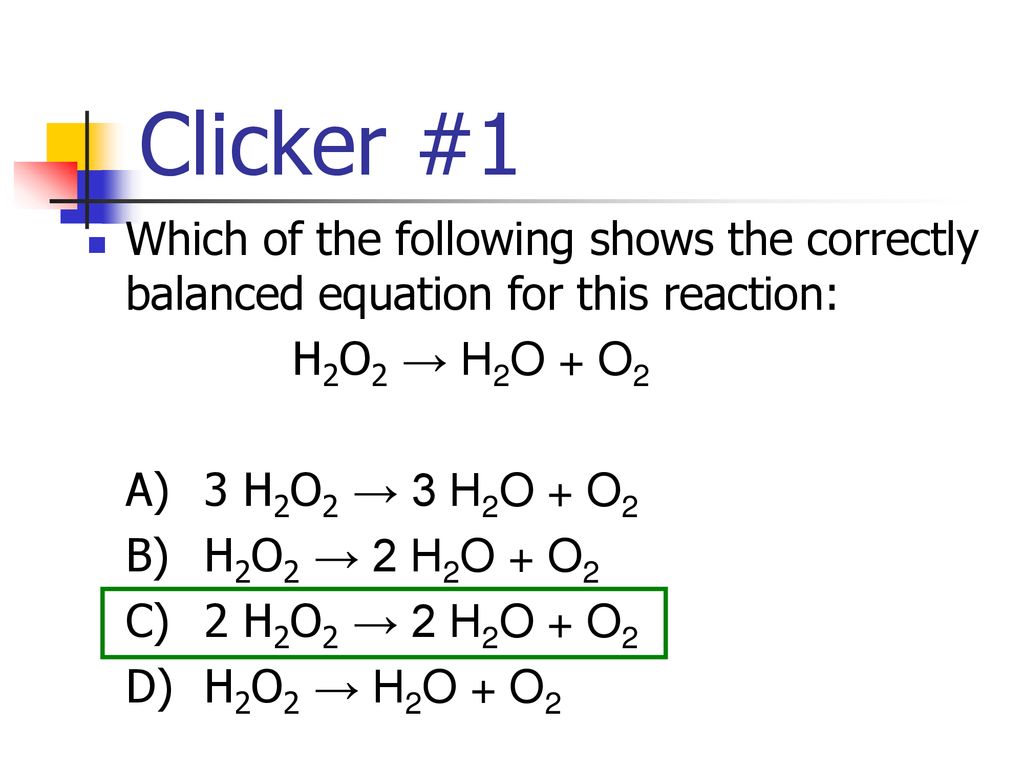
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download