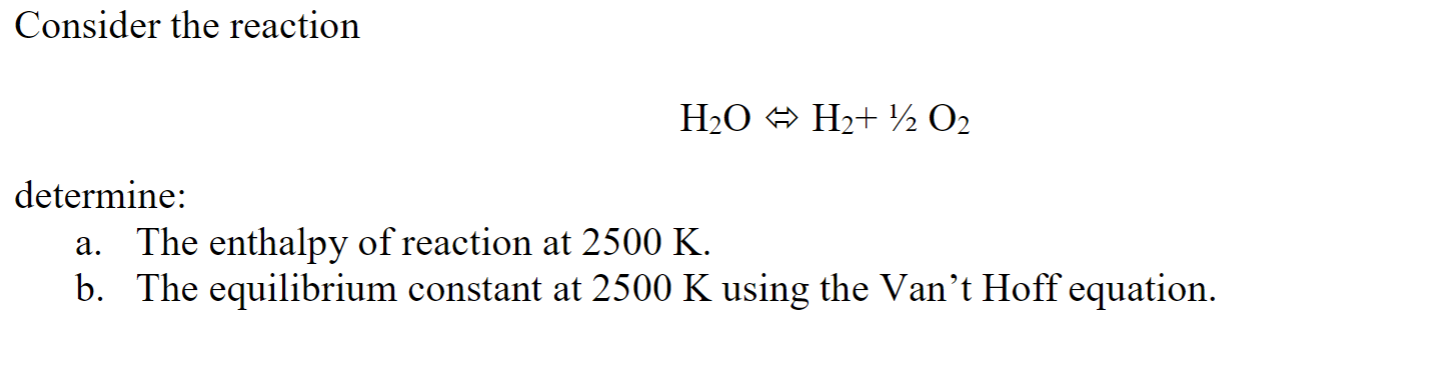31) The equilibrium constant the reaction H2O + CO) = H2(g) + CO2(g) is 0.44 1260K. The equilibrium constant the reaction 2H2(g) + 2C029) 7=2C0g + 2H2O(g) 1260 K is equal to

H2+O2=H2O balance the chemical equation @mydocumentary838. h2+o2=h2o balance the chemical equation. - YouTube

Schematic representation of small molecules (H2O, H2, and O2) involved... | Download Scientific Diagram
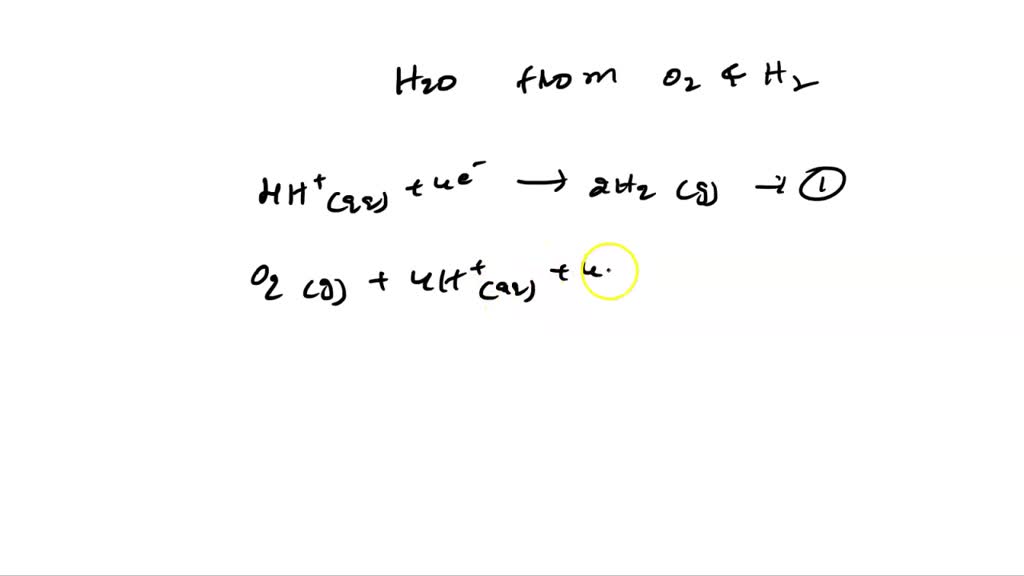
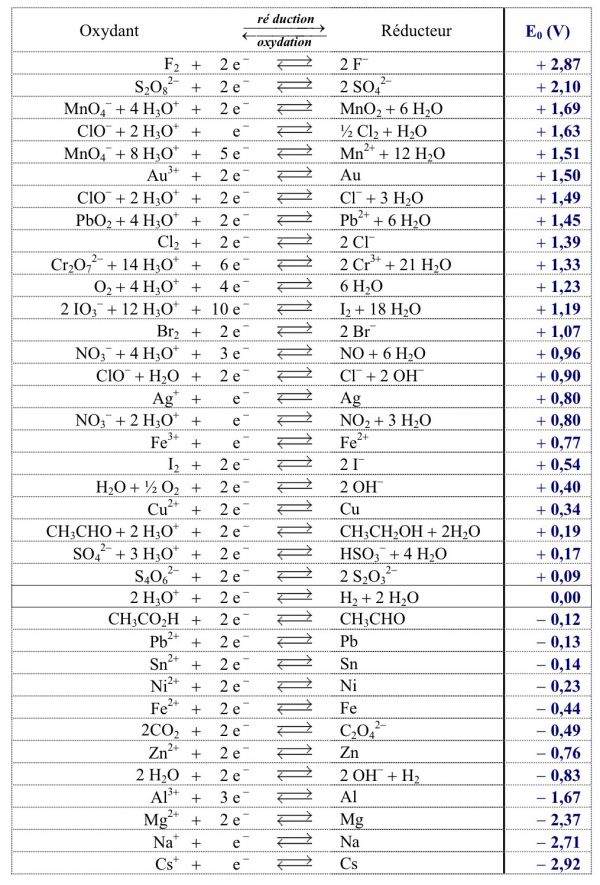
SOLVED: Express the formation of H2O from H2 and O2 in acidic solutions as the difference of two reduction half-reactions.

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)
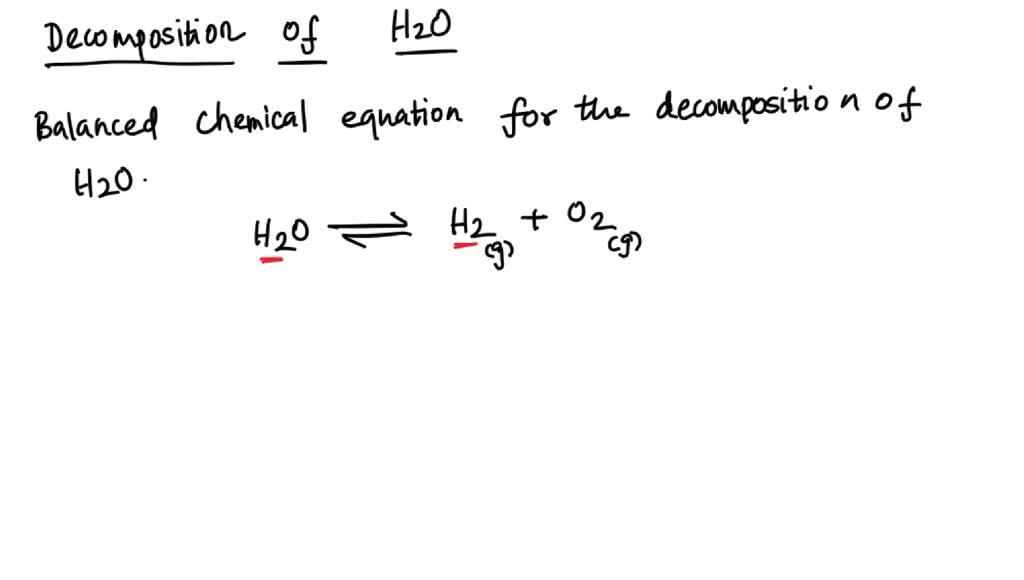
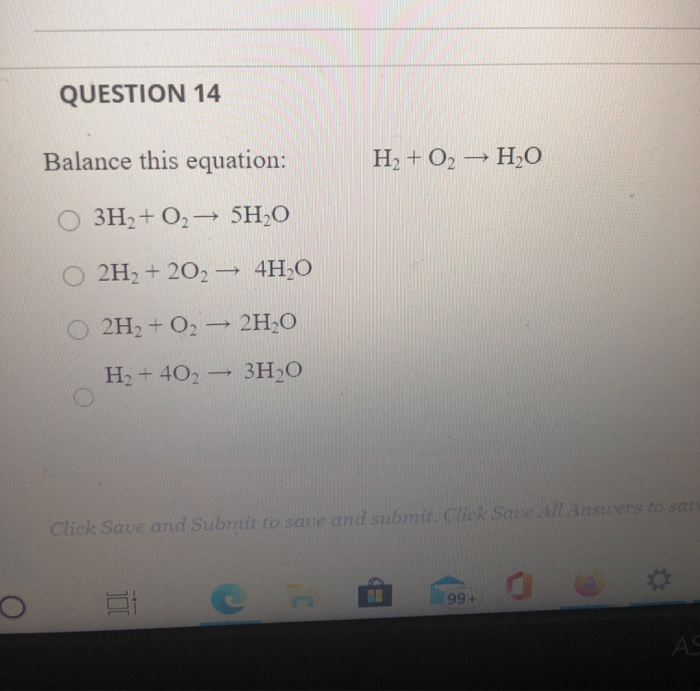
SOLVED: The decomposition of water into hydrogen gas H2 and oxygen gas O2 can be modeled by the balanced chemical equation A) H2 + O2 → H2O B) H2O → H2 +

PVT properties and diffusion characteristics of H2O/H2/CO2 mixtures in graphite nanoslits - ScienceDirect
n mole each of h2o h2 o2 r tken in closed container at temperature t if y mole of h2 r disasssociated at equillibrium n equillibrium pressure is p the cgt66gee -Chemistry -
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.