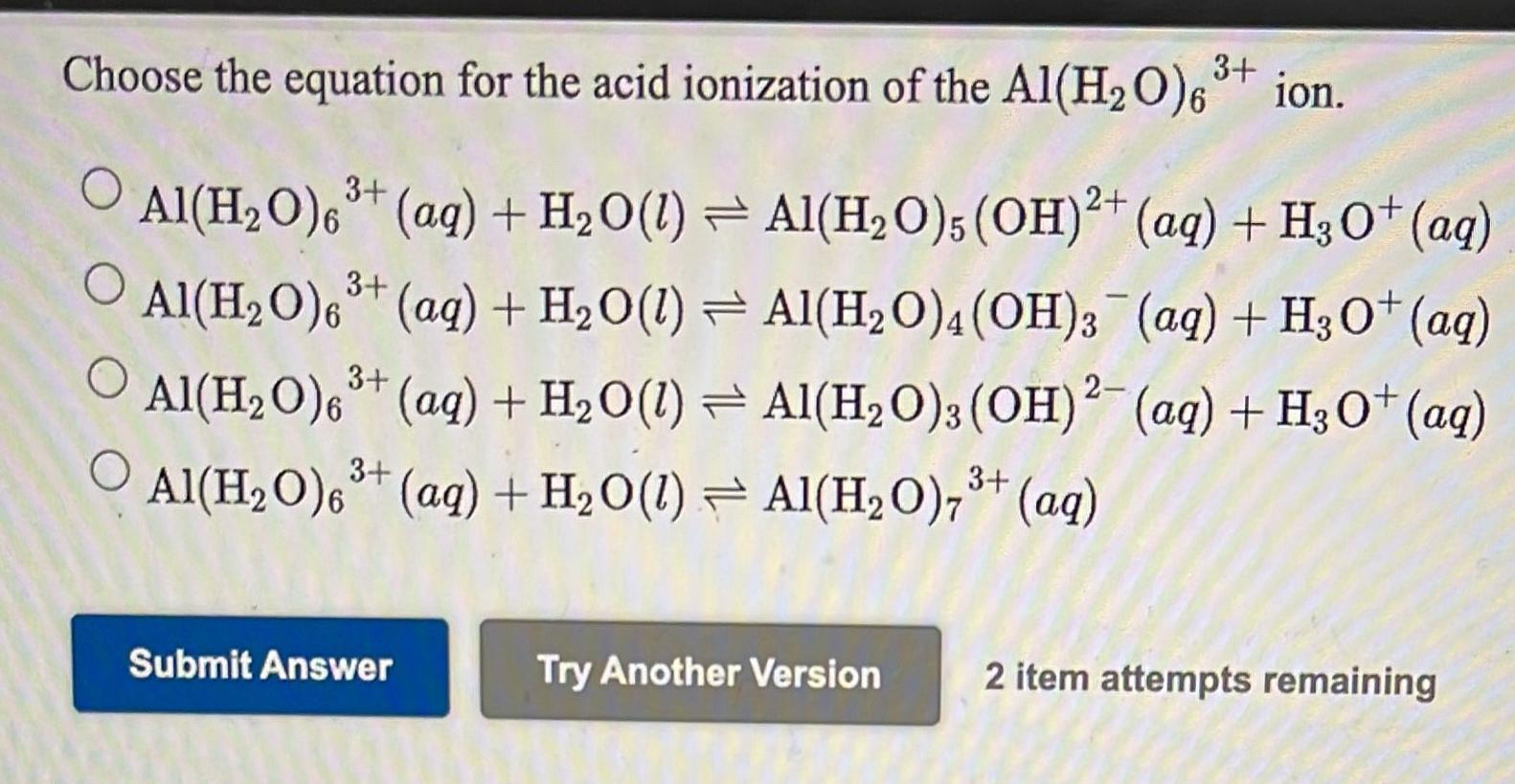
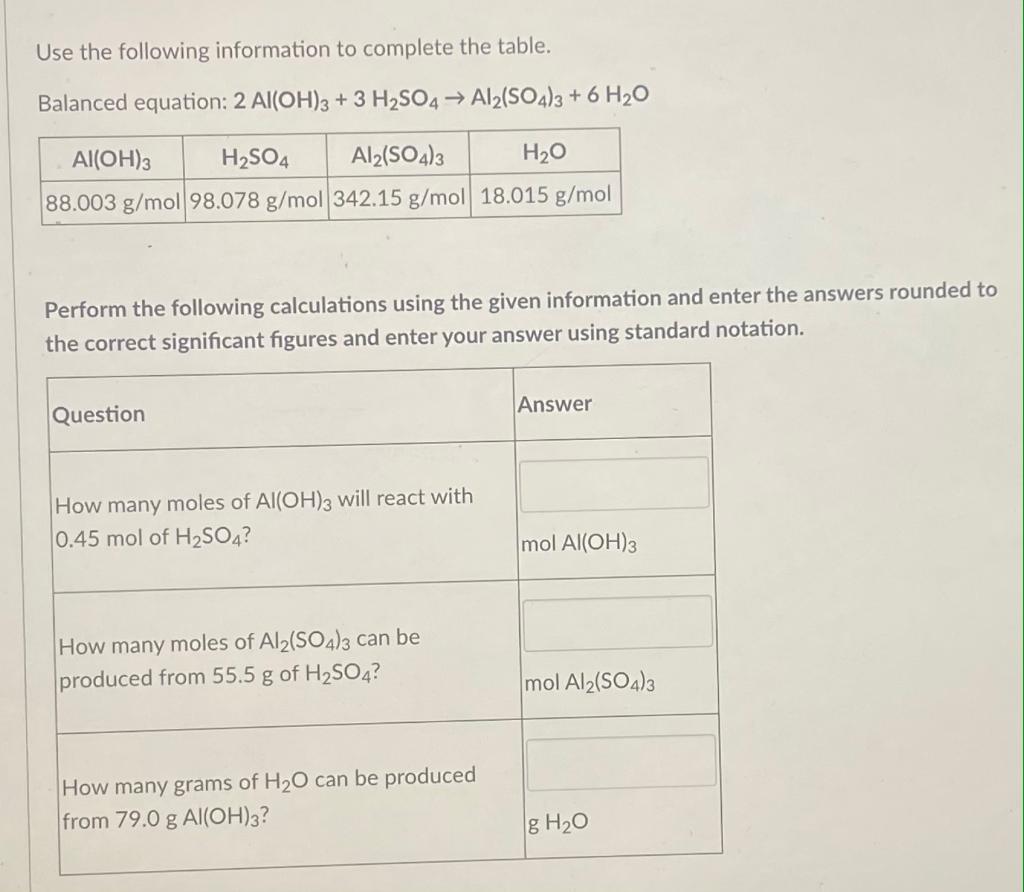
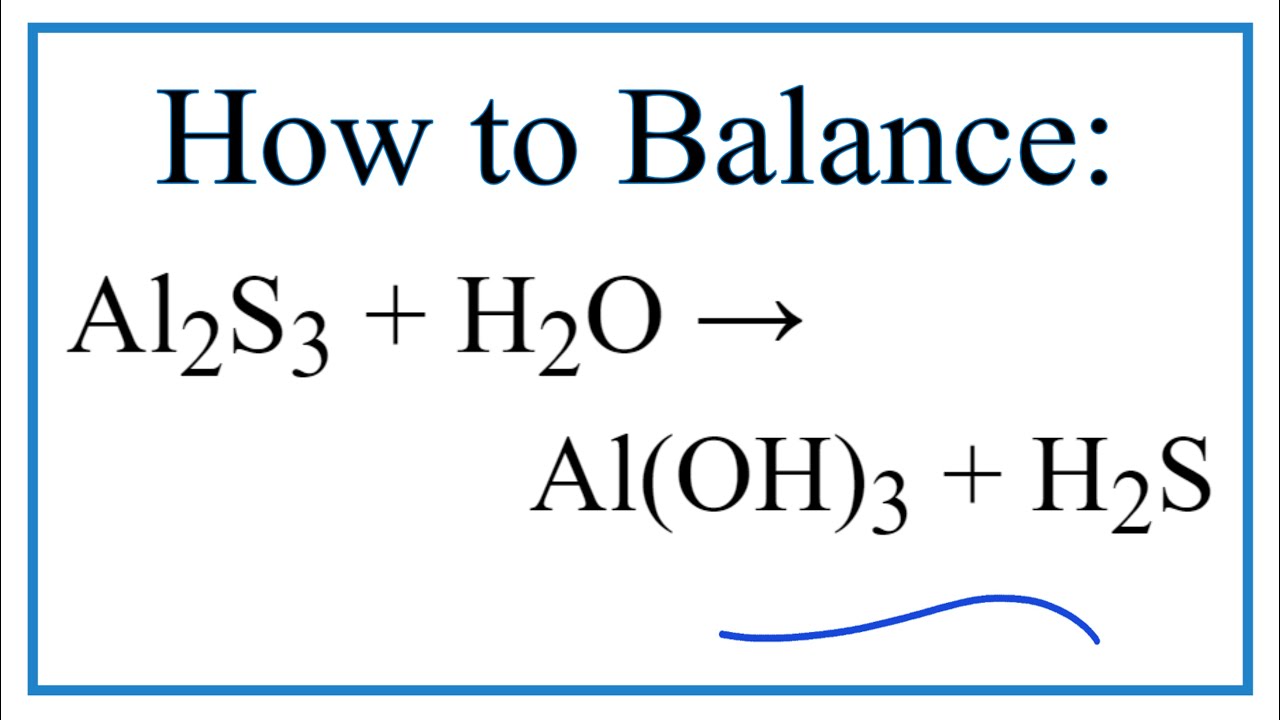
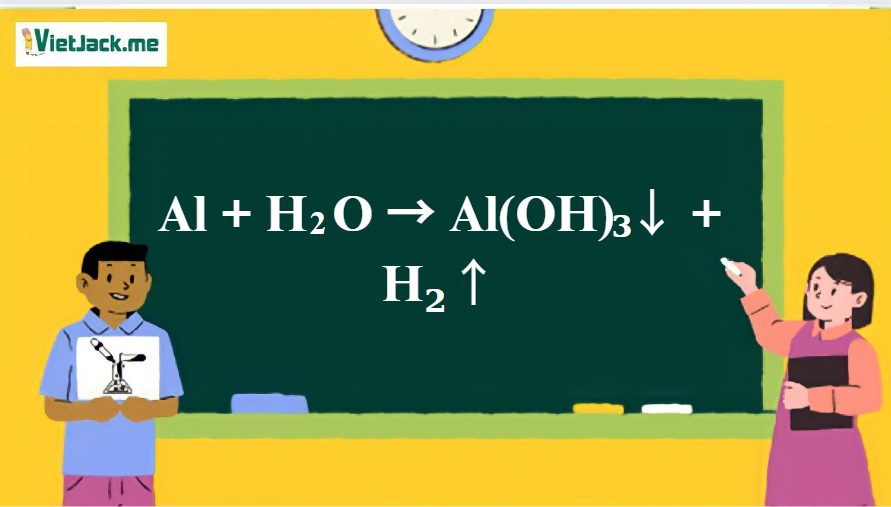SOLVED: Consider the following unbalanced equation: Al4C3 + H2O -> Al(OH)3 + CH4 How many moles of Al(OH)3 will be produced when 0.487 mol of CH4 is formed? 0.649 mol 0.162 mol 0.365 mol 0.487 mol
Al(H2O)6 3+ + H2O clusters with (A) single H-bond; (B and C) double... | Download Scientific Diagram
![SOLVED: In the reaction Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Bronsted-Lowry acid Lewis acid Bronsted-Lowry Base Lewis base SOLVED: In the reaction Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Bronsted-Lowry acid Lewis acid Bronsted-Lowry Base Lewis base](https://cdn.numerade.com/ask_previews/29288c5c-bf0a-455e-8d4b-2c5553f62277_large.jpg)
SOLVED: In the reaction Al3+ + 6H2O → [Al(H2O)6]3+, what does H2O act as? Bronsted-Lowry acid Lewis acid Bronsted-Lowry Base Lewis base
,... | Download Scientific Diagram Al(III)-superoxide complexes of the type [Al(O2·)(H2O)m(OH)n](q-1),... | Download Scientific Diagram](https://www.researchgate.net/publication/261803058/figure/fig10/AS:203162217652235@1425449055298/AlIII-superoxide-complexes-of-the-type-AlO2H2OmOHnq-1-formed-from.png)
Al(III)-superoxide complexes of the type [Al(O2·)(H2O)m(OH)n](q-1),... | Download Scientific Diagram