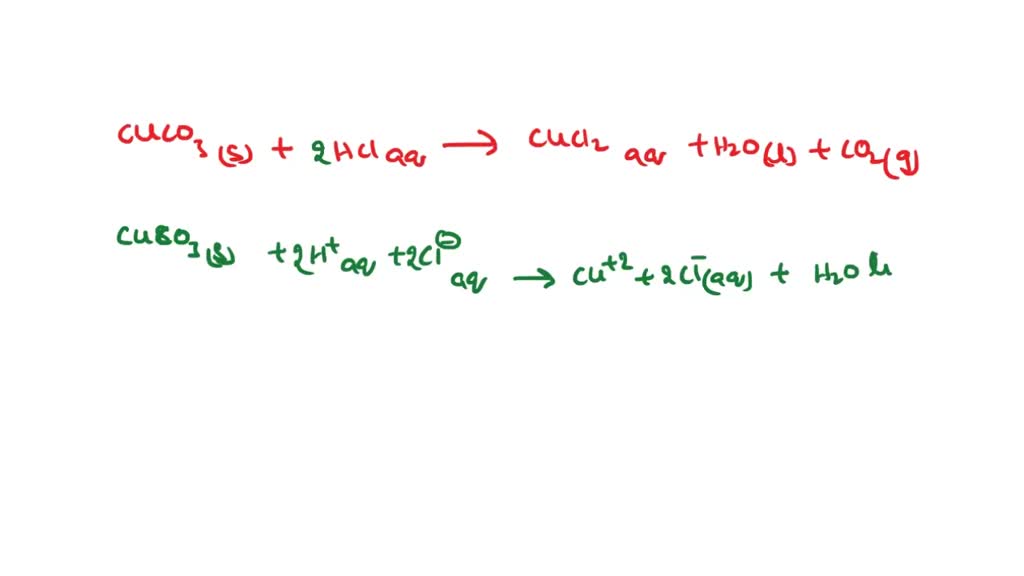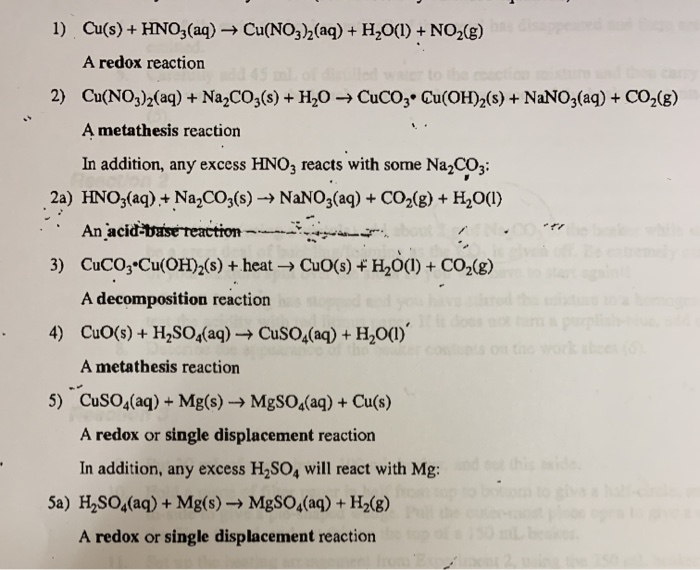La préservation du bois CAS 12069-69-1 de la poudre de carbonate de cuivre Cuco3 - Chine Poudre de carbonate de cuivre, de la poudre de carbonate cuivrique

Copper Carbonate,Senior Chemistry - Extended Experimental In-Industry News-Nickel Acetate,Cobalt Sulfate-Fairsky Industrial Co., Limited
%20carbonate%20(CuCO3)%20powder%20is%20added%20to%20a%20beaker%20with%2004M%20nitric%20acid%20(HNO3)%20Carbon%20dioxide%20(CO2)%20bubbles%20are%20produced-%20CuCO3%20%2B%20HNO3%20--%20Cu(NO3)2%20%2B%20H2O.jpg)
Bildagentur | mauritius images | Copper carbonate reacts with acid, 2 of 2. Copper(II) carbonate (CuCO3) powder is added to a beaker with 0.4M nitric acid (HNO3). Carbon dioxide (CO2) bubbles are

Poudre de bleu-vert d'alimentation de l'usine Cuco3 de base de carbonate de cuivre - Chine Poudre de carbonate de cuivre, de la poudre de carbonate cuivrique

CuO + H2CO3 –––> CuCO3 + H2O. Beautiful copper II carbonate ❤ | Teaching chemistry, Chemistry education, Science chemistry

Example: CuCO3(s) + H2SO4(aq) CuSO4(aq) CO2(g) + H2O Add the excess solid to the acid until no more reacts. Heating may be required Filter off the excess. - ppt download
Solved: Copper carbonate (CuCO_3) reacts with hydrochloric acid (HCl) according to this equation: [algebra]
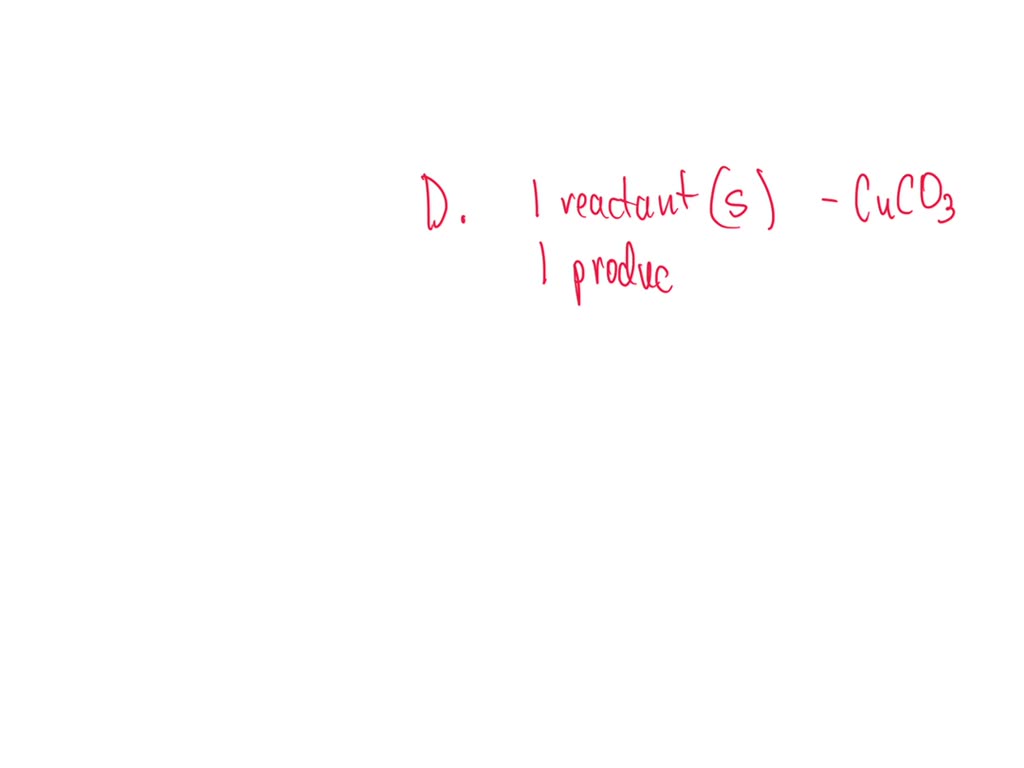
SOLVED: Question 34 Select the correct answer. Copper carbonate (CuCO3) reacts with hydrochloric acid (HCl) according to this equation: CuCO3(s) + 2HCl(aq) → CuCl2(aq) + H2O(l) + CO2(g). Which statement correctly describes
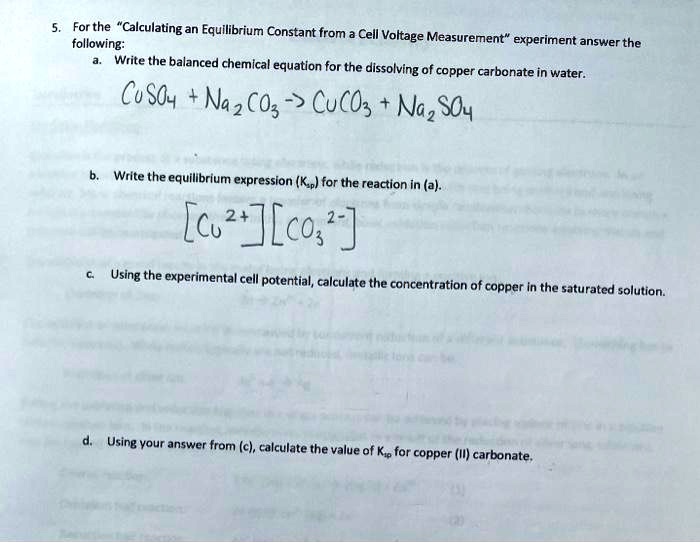
SOLVED: For the "Calculating an Equilibrium Constant from Cell Voltage Measurement" experiment, answer the following: Write the balanced chemical equation for the dissolving of copper carbonate in water: CuCO3 + H2O â†'
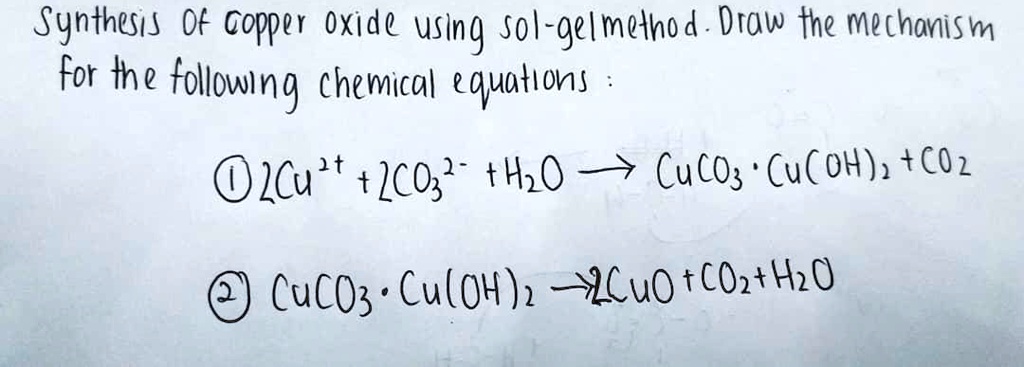


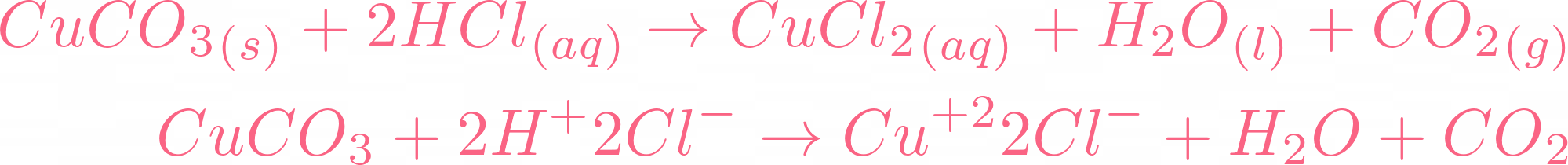

%20carbonate%20basic%20,%20CuCO3.CU(OH)2.H2O,%20%C4%90%C3%B4%CC%80ng(II)cacbonat%20baz%C6%A1%20,%20S%C6%A0N%20%C4%90%E1%BA%A6U%20,%20JHD%20CHEMICAL%20-%20Copy.jpg)