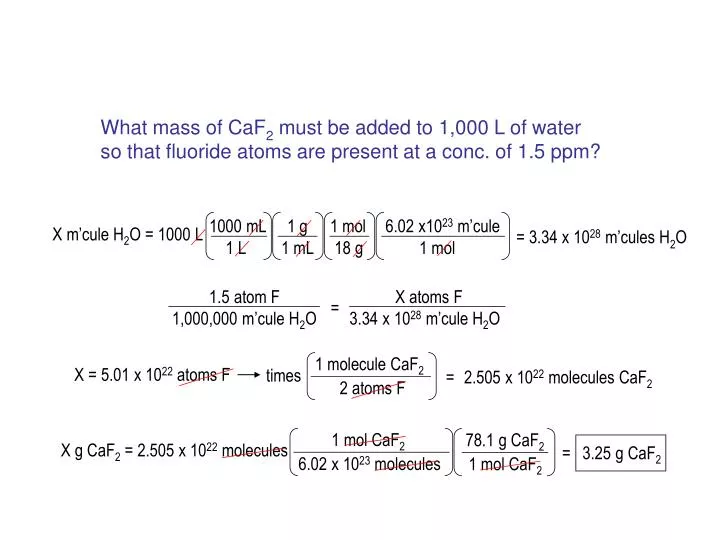
SOLVED: Consider the unbalanced chemical equation below: CaSiO3(s) + HF(g) â†' CaF2(aq) + SiF4(g) + H2O(l) Suppose a 34.4 g sample of CaSiO3 is reacted with 31.6 L of HF at 27.0
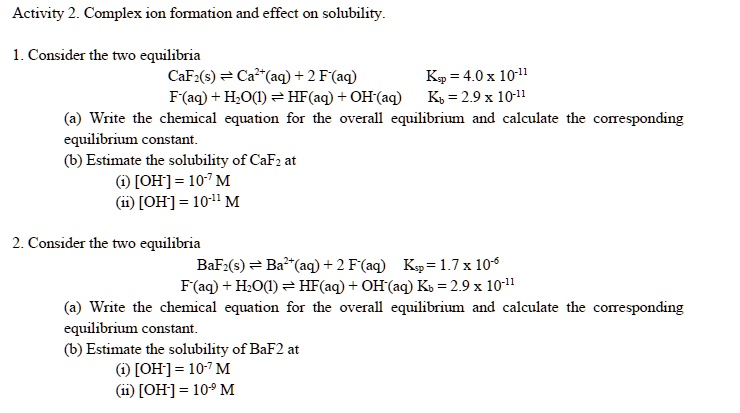
SOLVED: Activity 2: Complex Ion Formation and its Effect on Solubility Consider the two equilibria: CaF2(s) ⇌ Ca2+(aq) + 2F-(aq) Ks = 40 x 10^-11 F-(aq) + H2O(l) ⇌ HF(aq) + OH-(aq)
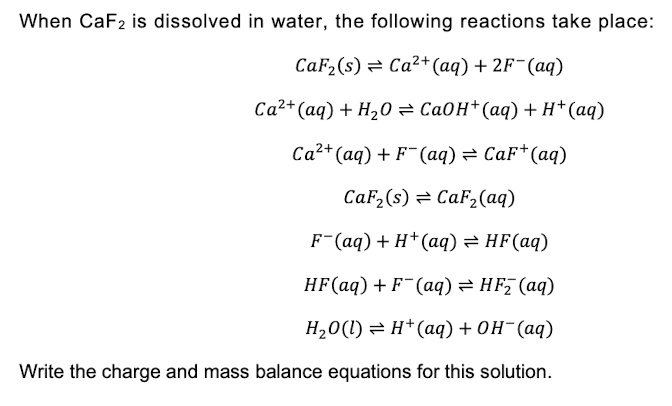
SOLVED: When CaF2 is dissolved in water, the following reactions take place: CaF2(s) = Ca2+(aq) + 2F-(aq) Ca2+(aq) + H2O = CaOH+(aq) + H+(aq) Ca2+(aq) + F-(aq) = CaF(aq) CaF2(s) = CaF2(aq)

Charge Reversal Behavior at the CaF2/H2O/SDS Interface as Studied by Vibrational Sum Frequency Spectroscopy | The Journal of Physical Chemistry B

Luminescence of Eu3+ Activated CaF2 and SrF2 Nanoparticles: Effect of the Particle Size and Codoping with Alkaline Ions | Crystal Growth & Design

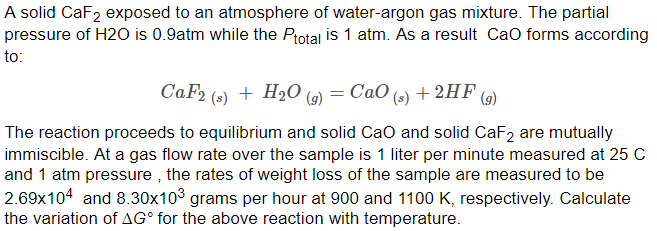







![Solved [2] (a) Consider the reaction: | Chegg.com Solved [2] (a) Consider the reaction: | Chegg.com](https://media.cheggcdn.com/study/0cd/0cd25e73-2e52-4764-8524-5f8d96b0fc58/image)