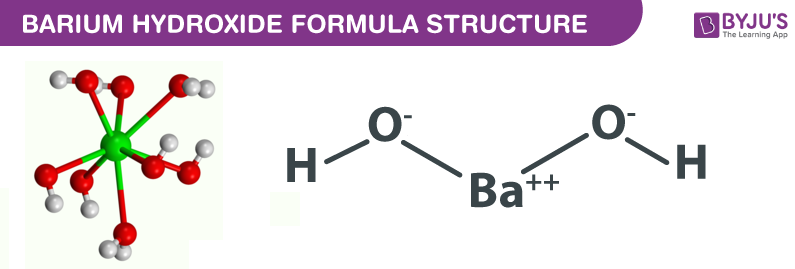
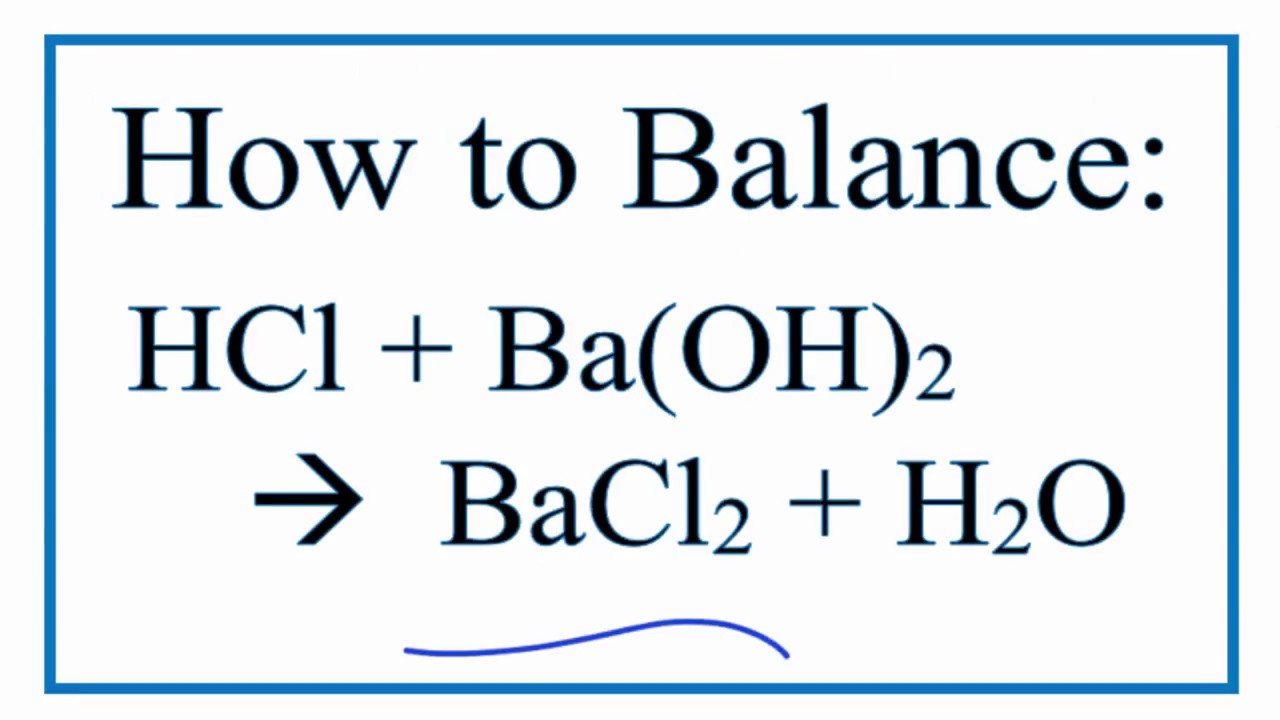
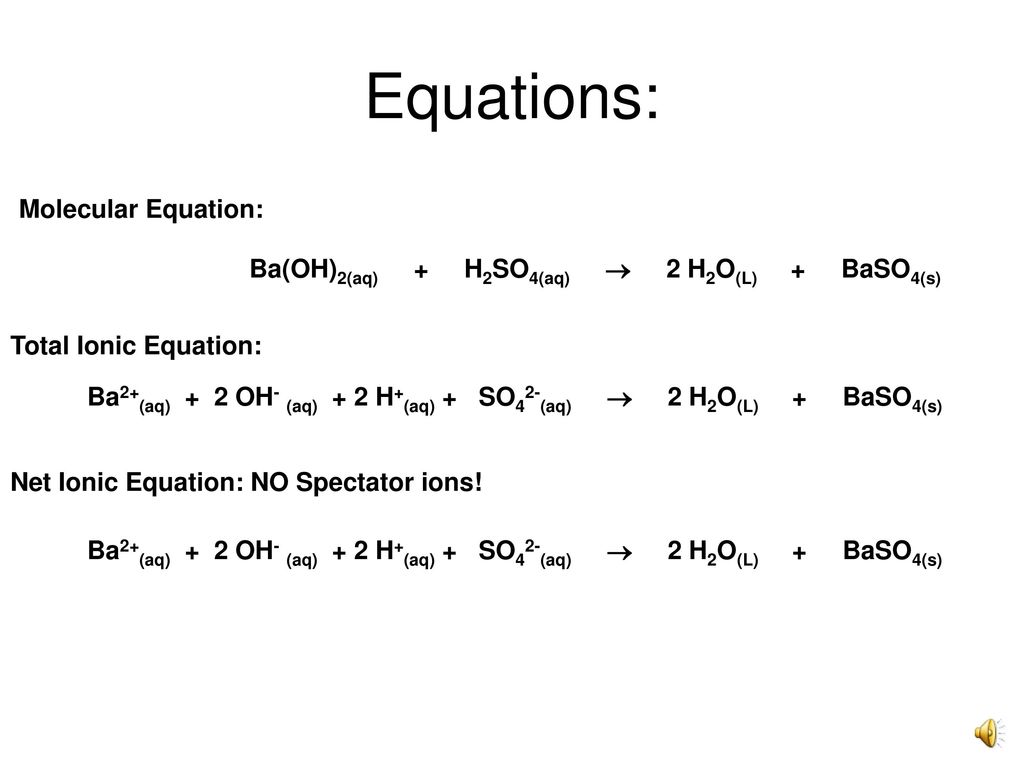
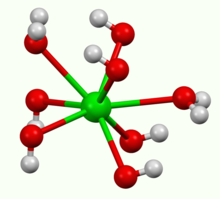
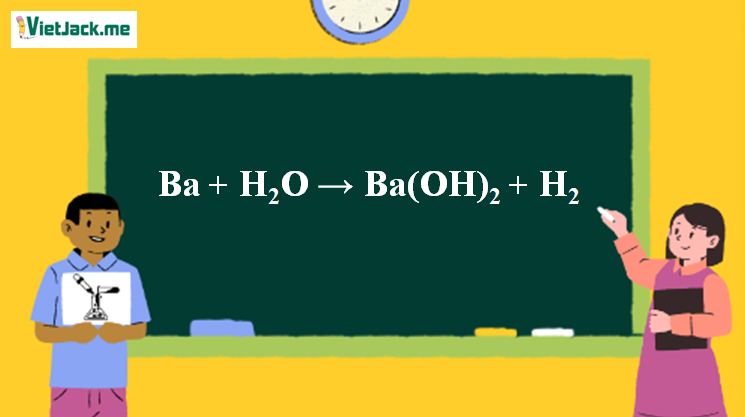How to Write the Net Ionic Equation for Ba(OH)2 + H2SO4 = BaSO4 + H2O (Note: it should be 2H2O) - YouTube

Ba + H2O =Ba(OH)2 +H2 Balanced Equation||Barium and Water=Barium hydroxide plus H2 Balanced Equation - YouTube

Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa
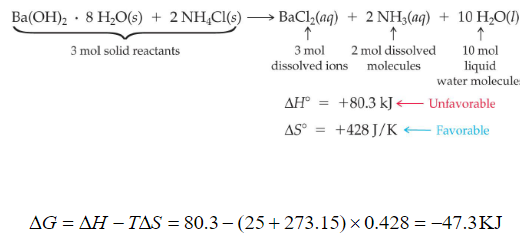
BALANCE THIS GIVEN EQUATION BY THE HELP OF THE ALTERNATE WAY OF BALANCING NOT THE TRADITIONAL WAY BY SHOWING THE APPROPRIATE STEPS ELABORATELY. EQN : Ba(OH)2 + NH4Cl —> BaCl2 + NH3 + H2O

Figure 5 from Ba(OH)2 Equilibria in the System Ba-O-H-F, With Application to the Formation of Ba2YCu3O6.5 + x From BaF2-Precursors | Semantic Scholar

Barium Hydroxide Monohydrate Ba (OH) 2. H2O - China Barium Hydroxide, Barium Hydroxide Monohydrate | Made-in-China.com
Acheter de l'hydroxyde de baryum? - Hydroxyde de baryum de la meilleure qualité à un prix avantageux chez Laboratoriumdiscounter